Wound healing is a biological process aimed at repairing and restoring damaged tissues within the body.[1] It is a natural and dynamic response that occurs in stages.[2] The wound healing process can be broadly divided into three main stages: Inflammation, proliferation, and remodeling, which comprise crucial processes such as inflammation, angiogenesis, extracellular matrix (ECM) remodeling, and cell migration.[3,4] In addition, the healing process involves complex interactions between various cell types and signaling pathways.[5,6] The quality of wound healing plays a key role in ensuring optimal recovery and restoring normal function in damaged tissues, encompassing aspects such as healing speed, minimal scarring, and restoration of tissue integrity.[7-9] The importance of wound healing quality is widely recognized and emphasized in clinical practice and scientific research. Parameters such as wound closure rate and the formation of tissue granulation are crucial in evaluating the speed of wound healing. Furthermore, ECM components, cell proliferation (proliferating cell nuclear antigen [PCNA]), and angiogenesis (cluster of differentiation 31 [CD31]) in skin tissue are essential indicators of wound healing quality.[10-12] Different types of cells, including fibroblasts and myofibroblasts, produce collagen (type I collagen alpha-1 chain [Col1a1] and type III collagen alpha-1 chain [Col3a1]), which is crucial for wound closure and tissue repair.[13,14]
The Wnt/b-catenin signaling pathway is a key mechanism involved in the wound healing process.[15] It plays a crucial role in regulating cell proliferation, migration, and differentiation, which are essential for efficient wound healing.[16] During wound healing, the activation of the Wnt/b-catenin pathway promotes the cell proliferation of epithelial cells at the wound edges, contributing to wound closure.[17,18] It also enhances the migration of endothelial cells toward the wound site, promoting the formation of granulation tissue and angiogenesis.[19] In addition, the Wnt/b-catenin pathway influences the differentiation of fibroblasts into myofibroblasts, which are crucial for wound contraction.[20]
Esculin is a natural compound found in plants such as horse chestnut and seven-leaf clover. Its antibacterial, anti-inflammatory, antioxidant, and angiogenic properties have been studied, hinting at its potential in wound healing.[21] Previous literature has reported that esculin can protect against renal ischemia-reperfusion injury by activating the PI3K/Akt pathway,[22] and escin, a related compound, can protect against lipopolysaccharide/DGal-induced liver injury by suppressing inflammation and oxidative reactions.[23] However, there is still a lack of data supporting the potential and mechanisms of esculin in wound healing. Given the complexity of the wound healing process and the numerous microenvironmental factors involved, this study aimed to explore whether esculin has a pro-healing effect on skin wound healing and its impact on healing quality. The study also investigated the potential of esculin in regulating the Wnt/b-catenin pathway during the wound healing process. These findings provide new insights into the molecular mechanisms underlying the potential of esculin in wound healing.
MATERIAL AND METHODS Experimental animalsNine 8-week-old and male C57BL/6JNifdc mice were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice were housed under constant conditions with a temperature of 22°C ± 2°C, humidity ranging from 50% to 60%, and a 12 h light/dark cycle.
Model establishment and drug administrationAll animals were anesthetized with 1.0% (w/v) pentobarbital sodium through intraperitoneal injection at a dose of 40 mg/kg, and the backs of the mice were shaved. Two full-thickness skin wounds were created using an 8 mm ONLY disposable punch (#P850, Acuderm, USA).[24] According to previous reports about esculin dosage,[23] the animals were randomly divided into three groups: The model group, the 20 mg/kg esculin group, and the 40 mg/kg esculin group, with three mice in each group. Esculin (#E8230, Beijing Solarbio, China) was administered through oral gavage. We observed the wound healing progress daily and took photographs on days 0, 7, 10, and 14. The wound area was calculated with Image Pro Plus Software (version 6.0, Media Cybernetics, USA) at each time point.[25]
Histopathological stainingThe mice were anesthetized and euthanized by intraperitoneal injections of pentobarbital sodium at a dose of 100 mg/kg. Appropriate amounts of wound skin tissue were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned into 5 μm-thick slices. Hematoxylin and eosin (H&E) staining (#G1120, Beijing Solarbio) was performed to observe the formation of wound granulation tissue and measure the thickness of the skin epithelium. Masson staining (#G1346, Beijing Solarbio) was used to assess the deposition of ECM in the tissue. Immunohistochemistry (IHC) staining was performed for the detection of Col1a1 (1:200 dilution, #A1352, ABclonal, China) and Col3a1 (1:200 dilution, #A3795, ABclonal) to evaluate the collagen content in the skin. The slides were deparaffinized, rehydrated, and treated with boiling citrate antigen retrieval buffer (sodium citrate buffer, 0.01 mol/L, pH 6.0) for 15 min. This was followed by blocking non-specific antibody binding with 5% goat serum (#SL038, Beijing Solarbio) at 37°C for 1 h. After overnight incubation with the primary antibody at 4°C, visualization was achieved using the streptavidinbiotin complex-peroxidase (rabbit Immunoglobulin G [IgG]) Kit (#SA1022, BOSTER, China). Images were captured using a Leica positive fluorescence microscope (THUNDER Imager 3D Tissue, Leica, Germany), and quantification was performed using Image-Pro Plus software for semi-quantitative evaluation of the results.
Immunofluorescence (IF)Paraffin-embedded sections of mouse skin were deparaffinized, rehydrated, and treated with boiling citrate antigen retrieval buffer (sodium citrate buffer, 0.01 mol/L, pH 6.0) for 10 min. The slides were blocked with 5% (v/v) goat serum for 1 h at 37°C and then incubated overnight at 4°C with rabbit anti-CD31 antibody (1:200 diluted in 1% [v/v] goat serum solution, #db15306, Diagbio, China) and rabbit anti-PCNA antibody (1:200 diluted in 1% [v/v] goat serum solution, #db11523, Diagbio). Cells cultured in glass cell creep were fixed in cold 4% (w/v) paraformaldehyde for 20 min and then incubated with 0.5% (v/v) Triton X-100 at room temperature for 10min. Cell creep was blocked with 5% (v/v) goat serum for 1 h at 37°C and then incubated overnight at 4°C with rabbit anti-PCNA antibody. The slides or cell creep were washed and incubated for 1 h with coraLite488-conjugated goat anti-rabbit IgG (1:200 diluted in 1% [v/v] goat serum, #SA00013-2, Proteintech, China). Finally, all samples were mounted with an antifade mounting medium containing diamidino phenylindole (#36308ES20, YEASEN, Shanghai, China). Images were captured and quantified using a Leica-positive fluorescence microscope (THUNDER Imager 3D Tissue, Leica, Germany).
Western blotAfter the tissue near the wound healing of the mouse skin was collected, the protein supernatant was extracted after homogenization in lysis buffer (#P0013B, Beyotime, Shanghai, China). The protein concentration was quantified by a bicinchoninic acid protein assay kit (#E112, Vazyme Technology, Nanjing, China), and the sample was prepared for Western blot. Western blot analysis was performed to detect the protein expression levels of the Wnt/b-catenin signaling pathway-related proteins, including Wingless-type MMTV integration site family, member 3A (Wnt3a) (1:1000 dilution, #db14992, Diagbio), glycogen synthase kinase 3 beta (GSK3b) (1:3000 dilution, #22104-1-AP, Proteintech), phosphorylated GSK3b (phosphorylated site Ser9, 1:3000 dilution, #67558-1-Ig, Proteintech), Snai1 (1:1000 dilution, #bs-1371R, Bioss, China), E-Cadherin (1:1000 dilution, #db6304, Diagbio), Vimentin (1:1000 dilution, #db11589, Diagbio), and b-catenin (1:5000 dilution, #66379-1-Ig, Proteintech). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:50,000 dilution, #60004-1-Ig, Proteintech) was used as an internal control. HRP-conjugated Affinipure Goat Anti-Mouse IgG (1:10000 dilution, #SA00001-1, Proteintech) and horseradish peroxidase-conjugated Affinipure Goat Anti-Rabbit IgG (1:10000 dilution, #SA00001-2, Proteintech) were used as secondary antibodies. The blots were developed using a highly sensitive enhanced chemiluminescence detection kit (#E411, Vazyme, China) and captured using a ChemiDoc XRS+ Chemiluminescence gel imager (BIORAD, USA). Protein bands were analyzed with the Image Lab software (version 6.1, BIO-RAD, California, USA), and protein expression was normalized to the expression levels of GAPDH.
Cell culture and assayThe NIH/3T3 cell line (#CL-0171, Pricella, Wuhan, China), fibroblast-like cells derived from mouse embryos, was cultured with 4.5 g/L D-glucose Dulbecco’s modified eagle medium (#C11995500BT, Gibco, USA) containing 10% fetal bovine serum (#10270106, Gibco) and 1% penicillin and streptomycin mixture (#CB010, Epizyme, Shanghai, China). The cell lines used in this study have all undergone short tandem repeat authentication and tested negative for mycoplasma. After starvation, NIH/3T3 cells in a six-well plate were treated with 0, 50, and 200 μM esculin for 24 h, collected, and sampled for further tests.[26] The effect of esculin in promoting cell proliferation was determined through PCNA-positive staining by IF. The Wnt/b-catenin signaling pathway-related proteins in NIH/3T3 cells after esculin treatment were determined by Western blot.
Statistical analysisOne-way analysis of variance (Tukey’s multiple comparisons test) was employed for comparisons among multiple groups. Prism version 9.0 software (version 8.0, GraphPad Software, San Diego, California, USA, https://www.graphpad-prism.cn/) was utilized for statistical analysis of the data. In vivo experiments involved three mice in each group, whereas in vitro experiments involved three replicates in each group. All data were expressed as mean ± standard deviation. P < 0.05 was considered statistically significant.
RESULTS Esculin accelerates wound healing in miceTo evaluate the potential of esculin in improving skin wound healing in mice, Figure 1a presents a series of representative wound images captured on days 0, 7, 10, and 14 during different treatment periods. As shown in Figure 1b, compared with the model group, the esculin-treated group exhibited significant improvements in skin wound closure rates in C57BL/6 mice on days 7, 10, and 14 (P < 0.05). Specifically, on day 7, the wound closure rate of the group receiving 40 mg/kg esculin demonstrated a significant increase by 17.69% compared with that of the group receiving 20 mg/kg (P < 0.05, 76.893% ± 3.547% vs. 65.337% ± 6.456%). However, on days 10 and 14, the wound closure rate showed no significant difference between the group receiving 20 and 40 mg/kg (P > 0.05).
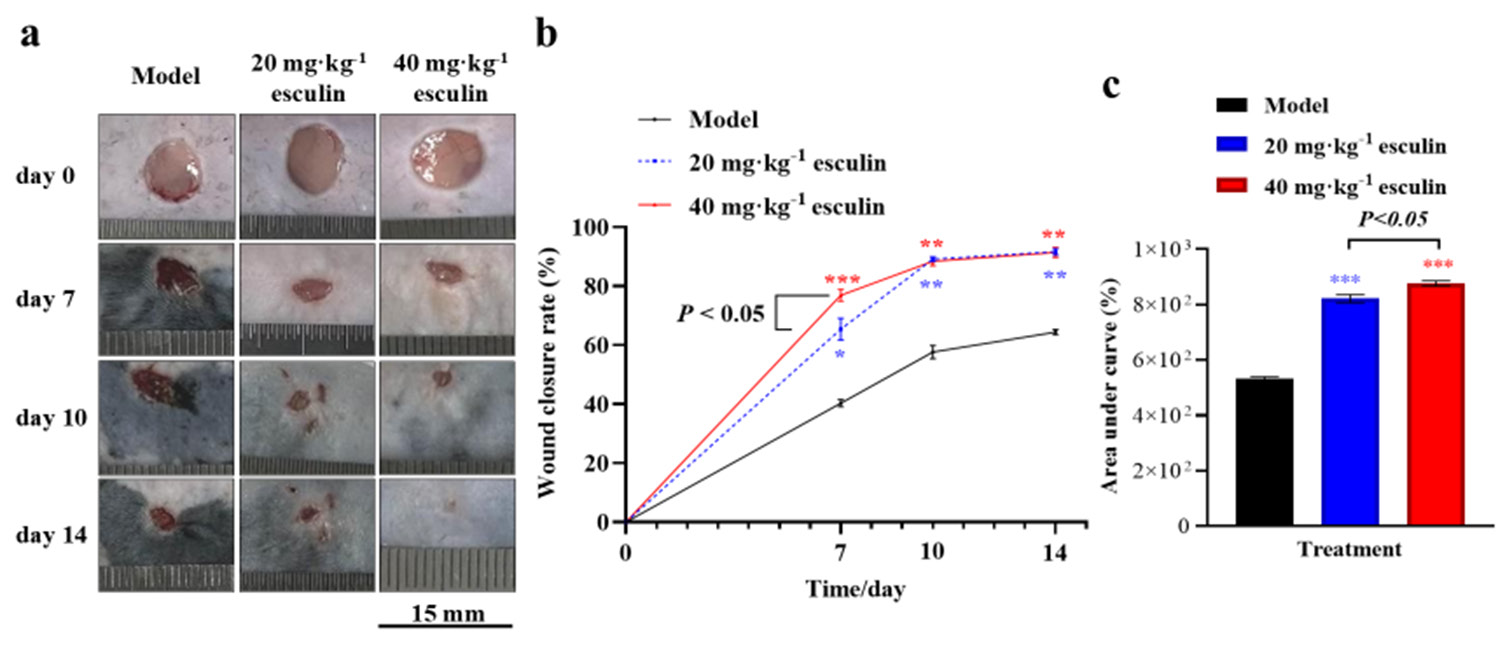
Export to PPT
Furthermore, analysis of the area under the curve for healing rates revealed that the 20 and 40 mg/kg esculin-treated groups promoted the wound closure rate compared with the model group ([Figure 1c], P < 0.001, 876.6% ± 16.2% and 821.8% ± 25.1% vs. 531.9% ± 13.0%). In addition, the 40 mg/kg esculin-treated group exhibited an overall improvement of 6.67% in promoting the wound closure rate in mice compared with the group receiving 20 mg/kg ([Figure 1c], P < 0.05).
Esculin enhances granulation tissue formation and epithelial regenerationMoreover, histological analysis was conducted through H&E staining on skin tissues from each group on day 14 to evaluate the improvement in granulation tissue formation in the esculin-treated groups. The results confirmed that the esculin-treated groups exhibited significant advantages in histopathological repair [Figure 2a]. Compared with the model group, the 40 mg/kg esculin-treated group showed an intact epithelium, with orderly arranged collagen in the dermis. In addition, the peripheral region was well-integrated into the surrounding native skin tissue, and numerous hair follicles were observed. By contrast, the dermis in the 20 mg/kg esculin-treated group still exhibited defects.
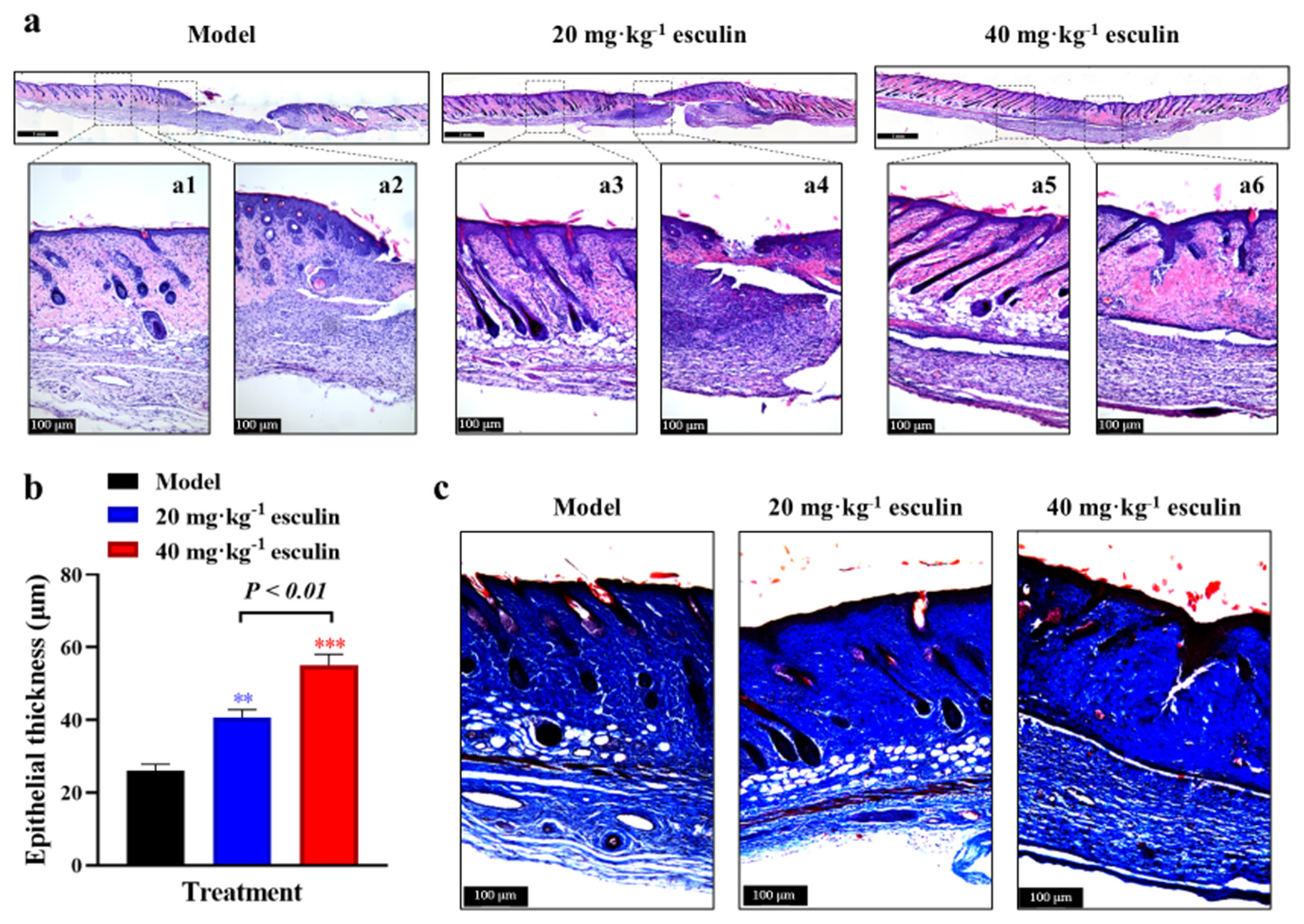
Export to PPT
To assess the effect of esculin on epithelial regeneration during wound healing, we measured the thickness of the epithelium on day 14. As shown in Figure 2b, the 20 and 40 mg/kg esculin-treated groups exhibited significantly thicker epidermal layers than the model group (P < 0.01, 40.67 ± 5.346 μm and 55.09 ± 7.437 μm vs. 26.05 ± 4.371 μm). In addition, the 40 mg/kg esculin-treated group exhibited an overall improvement of 35.46% in the 20 mg/kg esculin-treated group (P < 0.01). This finding suggested that esculin treatment effectively promoted epithelial regeneration, contributing to rapid wound healing.
Esculin promotes collagen deposition in wound tissueCollagen, as the primary component of the ECM, provides structural support to tissues and plays a crucial role in wound healing. Masson staining revealed that esculin significantly promoted the deposition of total collagen, visualized as blue staining, in the skin tissue of the wound area [Figure 2c]. Given that type I and type III collagen play key roles in the formation of new ECM, IHC staining was used to assess the levels of Col1a1 and Col3a1 proteins. As shown in Figure 3a-d, compared with the model group, significant increases in Col1a1 and Col3a1 protein levels were observed in the esculin-treated groups on day 14 (P < 0.05 or P < 0.01). However, the 40 mg/kg esculin-treated group only exhibited a slight increase in the expression of Col1a1 and Col3a1 protein than the 20 mg/kg esculin-treated group, and the difference was not significant (P > 0.05).

Export to PPT
Esculin induces cell proliferation and angiogenesisTo evaluate the proliferative effects, we assessed the expression of PCNA in the tissue on day 14. IF staining revealed significantly higher PCNA expression in the 20 and 40 mg/kg esculin-treated groups compared with that in the model group (1.531- and 1.554-fold, respectively, P < 0.01, [Figure 4a and b]). Newly formed blood vessels provide a network of oxygen and nutrient supply to the healing tissue, which is crucial for the survival and function of cells involved in wound healing. IF staining for CD31 demonstrated that esculin significantly promoted angiogenesis in the skin wound, resulting in a significant increase in CD31 positive staining in the 20 and 40 mg/kg esculin-treated groups compared with the model group (1.373- and 1.425-fold, respectively, P < 0.05, [Figure 4c and d]). However, compared with the 20 mg/kg esculin-treated group, the 40 mg/kg esculin-treated group exhibited no statistical significance in the expression levels of PCNA and CD31 proteins (P > 0.05).
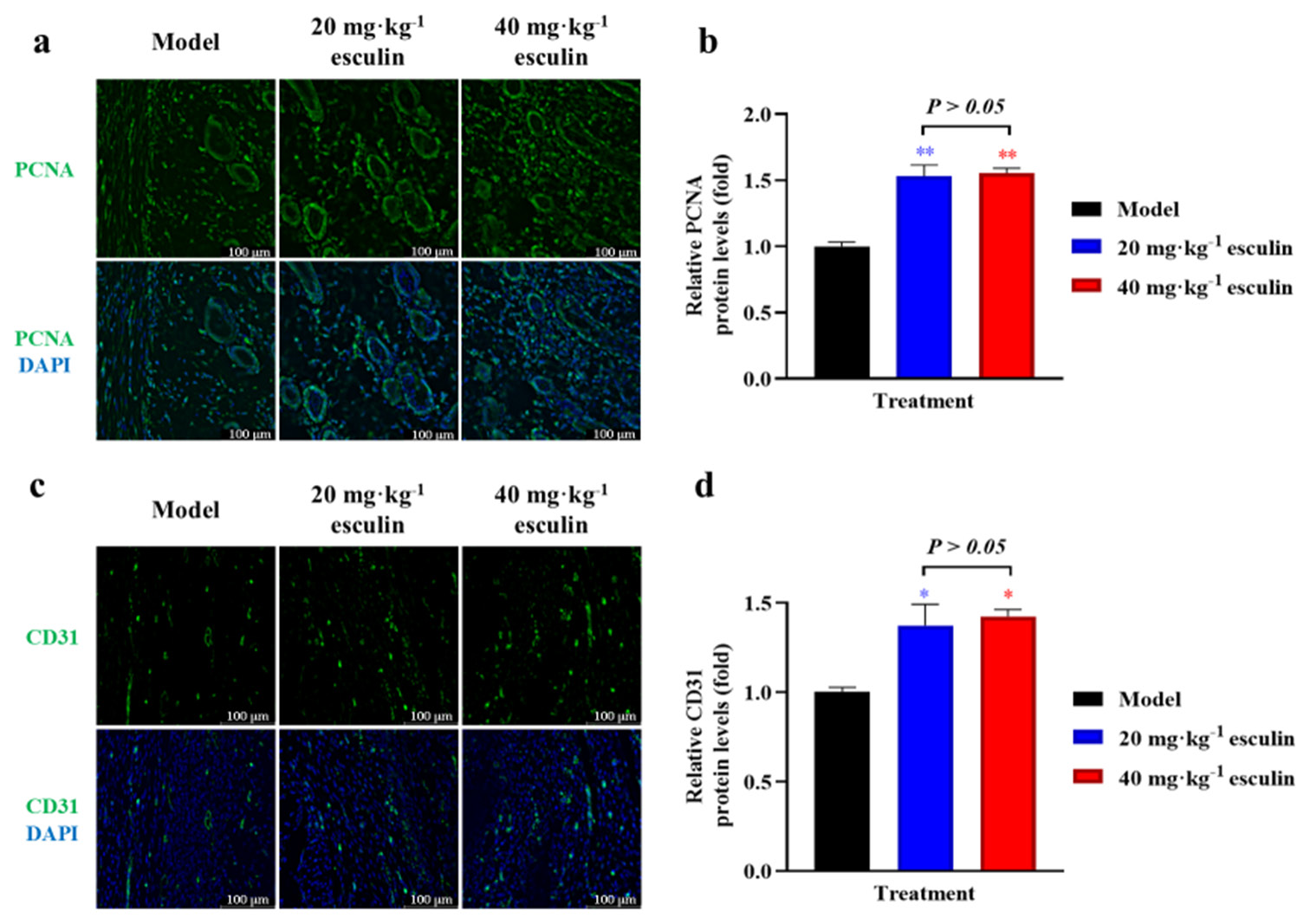
Export to PPT
Esculin significantly activates the Wnt/β-catenin signaling pathwayIn this study, we aimed to investigate whether esculin exerts its wound-healing effects through the Wnt/b-catenin signaling pathway. Therefore, we examined the expression levels of proteins associated with the Wnt/b-catenin signaling pathway in the wound tissue. As shown in Figures 5a and b, compared with the model group, the esculin-treated groups significantly upregulated the protein expression levels of Wnt3a, GSK3b, Snail, E-cadherin, Vimentin, and b-catenin in the mouse wounds (P < 0.05 at least). Furthermore, the 40 mg/kg esculin-treated group exhibited an overall improvement of 69.6% in protein expression of Wnt3a in mice compared with the group receiving 20 mg/kg ([Figure 5b], P < 0.01). The transmission of the Wnt pathway relies on GSK3b, and Western blot results demonstrated that esculin significantly promoted the phosphorylation level of GSK3b ([Figure 5], P < 0.05). These findings confirmed that esculin significantly upregulated and activated the Wnt/b-catenin signaling pathway during the wound healing process in mice.
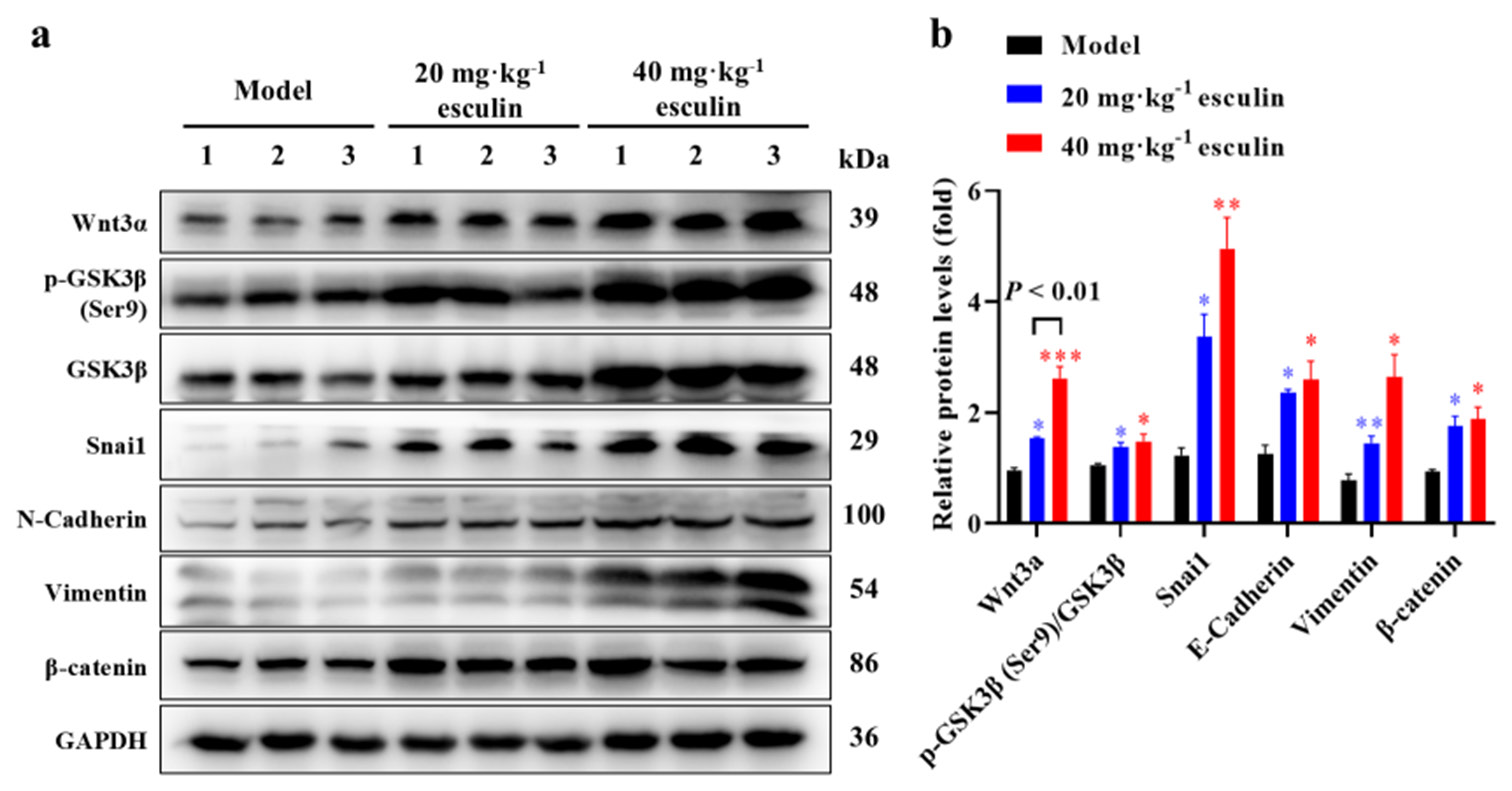
Export to PPT
Esculin significantly promotes fibroblast cell proliferation and activation of the Wnt/b-catenin signaling pathwayIn the results depicted in Figure 4a, we observed that the positive staining for PCNA was predominantly concentrated in the dermal layer and around the hair follicles in the esculin-treated group. Considering that fibroblasts are the primary constituent cells of the dermal layer, we hypothesized whether esculin exerts a proliferative effect on fibroblasts. As illustrated in Figures 6a and b, compared with the control group, the 50 and 200 μM esculin-treated groups significantly upregulated the mean intensity of PCNA positive staining in NIH/3T3 cells (P < 0.05 at least). Furthermore, compared with the 50 μM esculin-treated group, the esculin intensity of PCNA positive staining in the 200 μM esculin-treated NIH/3T3 cells increased by 1.26-fold ([Figure 6b], P < 0.05). Considering that the Wnt/b-catenin signaling pathway is involved in promoting fibroblast proliferation, we examined the expression levels of proteins related to the Wnt/b-catenin signaling pathway in NIH/3T3 cells treated with esculin. Western blot results revealed that the protein expression levels of Wnt3a, Snail, N-Cadherin, Vimentin, and b-catenin in NIH/3T3 cells, as well as the phosphorylation level of GSK3b (Ser9), were significantly upregulated after 50 and 200 μM esculin treatment ([Figure 6c and d], P < 0.05 at least) when compared with the control group.
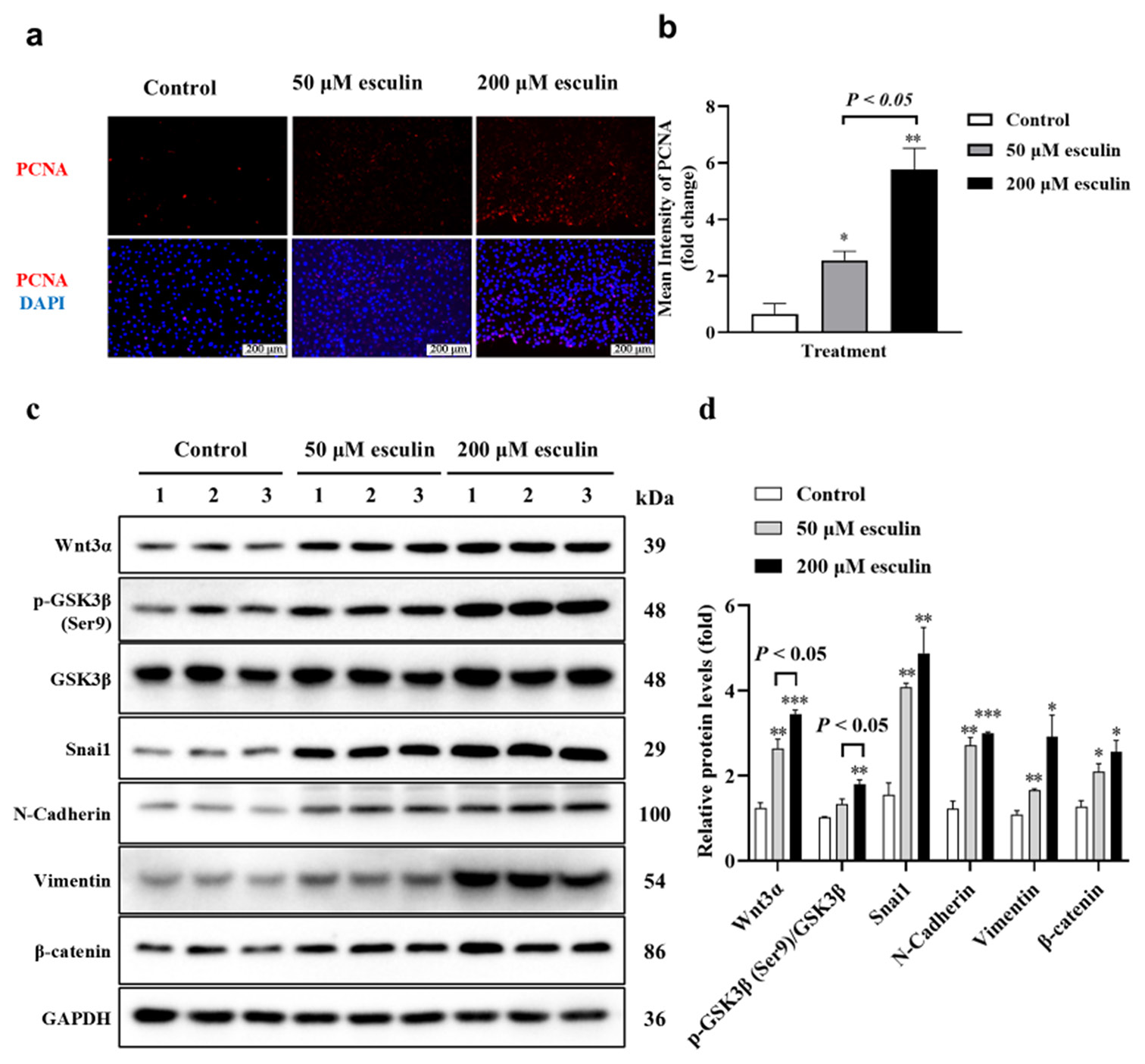
Export to PPT
DISCUSSIONWound healing is a complex biological process that involves a series of interconnected events aimed at repairing damaged skin tissue.[4] The quality of wound healing refers to the degree and effectiveness of the healing process, encompassing factors such as healing speed, appearance of scars, and restoration of normal skin function.[3,27,28] In this study, we comprehensively demonstrated that 20 and 40 mg/kg esculin treatment significantly promoted skin wound healing in mice from multiple aspects.
Initially, healthy granulation tissue, composed of neovascularization, fibroblasts, and ECM filling the wound bed, serves as a scaffold for tissue regeneration and provides a foundation for re-epithelialization. Our results confirmed that esculin significantly promoted the formation of granulation tissue, enhanced the speed and thickness of epithelialization, and accelerated wound closure. However, in terms of dosage, 40 mg/kg esculin treatment only showed a slight advantage in promoting wound healing on day 7, so the difference between the two doses must be increased in the future. Moreover, on day 14, the wounds of the model group still showed significant unhealed areas, which easily affected the integrity of the experimental skin and interfered with the experimental results.
During the wound-healing process, collagen is synthesized and organized to restore skin integrity.[29,30] Correct collagen synthesis and remodeling are crucial for achieving strong and flexible scars, thereby improving the quality of healing. Col1a1 and Col3a1 play different physiological functions in the strength and toughness of the later stage of skin wound healing. However, in our study, esculin significantly promoted the expression of both collagen proteins. Thus, our findings only confirmed that esculin treatment significantly increased collagen deposition in the dermis. Unfortunately, our results could not fully reflect the relative difference between the two kinds of collagen, omitting the difference in the final quality of wound healing. This study also indicated that the healing time of mice in the experiment was insufficient to accurately reflect the skin healing process.
Cell proliferation and angiogenesis are two critical processes in skin wound healing that play essential roles in achieving successful and effective tissue repair.[31] Cell proliferation is responsible for the rapid growth and multiplication of cells during wound healing, whereas angiogenesis involves the formation of new blood vessels, crucial for supplying oxygen, nutrients, and immune cells to the wound. Our results demonstrated that esculin administration significantly increased the expression of PCNA in the epidermis and dermis. The vascular marker CD31 is primarily distributed in the dermis, and esculin treatment significantly promotes the process of angiogenesis. However, as a result of limitations in sample size and timing, this study did not investigate the dynamic regulation of esculin on inflammatory responses during skin wound healing, which remains a future research direction.
Another contribution of this study is the discovery that esculin potentially promoted cell proliferation by upregulating proteins related to the Wnt/b-catenin pathway and activating the phosphorylation levels of these proteins. However, the proliferating cells during wound healing include fibroblasts and keratinocytes, among others.[32] During proliferation, fibroblasts proliferate and migrate to the wound bed, synthesizing and depositing collagen to strengthen the wound.[33,34] Keratinocytes migrate from the wound edges to close the wound and restore the protective barrier function of the skin.[6,35] In this study, the results presented in Figure 6 demonstrated that esculin significantly enhanced the proliferative capacity of mouse fibroblasts, accompanied by the activation of the Wnt/b-catenin signaling pathway. Further investigation is required to elucidate the effects of esculin on other aspects of fibroblast function, as well as its impact on the functionality of other types of cells.
SUMMARYThis study clarified the potential therapeutical effect of esculin on promoting skin wound healing, which might benefit from accelerated granulation tissue formation, epithelial regeneration, collagen deposition, cell proliferation, and vascular regeneration through regulating the Wnt/b-catenin signaling pathway in mice. Our results provide new insights for the development of esculin, directing its clinical application.
AVAILABILITY OF DATA AND MATERIALSThe data and materials that support the findings of this study are available from the corresponding author on reasonable request.
ABBREVIATIONSCD31: Cluster of differentiation 31
Col1a1: Type I collagen alpha-1 chain
Col3a1: Type III collagen alpha-1 chain
GSK3β: Glycogen synthase kinase 3 beta
PCNA: Proliferating cell nuclear antigen
p-GSK3β: Phosphorylated glycogen synthase kinase 3 beta
Snail1: Snail family transcriptional repressor 1.
Wnt3α: Wingless-type MMTV integration site family, member
AUTHOR CONTRIBUTIONSMX and MSZ: Designed the study, all authors conducted the study; JJW, JMW, and HZW: Collected and analyzed the data; MX, MSZ, and XTX: Participated in drafting the manuscript, and all authors contributed to critical revision of the manuscript for important intellectual content. All authors gave final approval of the version to be published. All authors participated fully in the work, take public responsibility for appropriate portions of the content, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or completeness of any part of the work are appropriately investigated and resolved.
Comments (0)