Dear Editor,
Psoriasis is a chronic inflammatory dermatosis with immune-mediated polygenic defects. Erythrodermic psoriasis (EP) is one of its severe variants which is not uncommon in Asian populations1 and has a high mortality rate.2 Recently, several authors have highlighted the important role of infections in its occurrence and development. However, few studies exist to identify the risk-factors associated with infection in these patients, especially studies targeting severe EP, clinical features, and laboratory markers associated with infection. This project investigated clinical features, laboratory tests, and related factors of EP patients with systemic infections by retrospectively analysing their medical records, which were retrieved from the Affiliated Hospital of Xuzhou Medical University. The probability of EP patients merging with infections is high and types are diverse. Joint pain/pustules, smoking history, hyperglycemia, and hypoproteinemia are related factors for EP combined with infection. C-reactive protein, systemic immune inflammation index, α-1 acidic glycoprotein, N-terminal B-type natriuretic peptidogen, albumin, cholinesterase, and total cholesterol are new indicators for diagnosing EP with infection, and the regression predicted values performs better.
The hospital information system (HIS) of the Department of Dermatology of the Affiliated Hospital of Xuzhou Medical University was searched and all relevant medical records for EP were reviewed from January 2012 to December 2022. Finally, we included 105 inpatients with EP and concomitant infection in our study. Our study has been approved by the Medical Ethics Committee of Xuzhou Medical University Affiliated Hospital with opinion number XYFY2022-KL454-01.
This study used retrospective analysis methods to collect data from all included patients, including basic demography, clinical characteristics, and laboratory findings. We included the results of the first test only in patients who underwent multiple tests for the same item. All data was organised for statistical analysis using Software SPSS26.0.
Chi-square test, continuous corrected chi-square test, independent sample t-test, corrected t-test, Mann–Whitney U test, and unconditional logistic regression were used. The test level α was defined as 0.05 and P<0.05 was considered statistically different and P<0.01 was considered to be significantly different in the distribution between both groups. Receiver Operating Characteristic (ROC) curve was analysed and drawn by the software GraphPad Prism 9.
Excluding 32 suspected infections, 105 hospitalised patients with EP were finally included in this study with a total of 62 patients with systemic infection and 43 patients without systemic infection with an overall infection rate of 45.3%. Various types of infections may complicate EP, respiratory infections being the most frequent [Table 1].
Table 1: Patients with erythrodermic psoriasis and systemic infection.
Type/pathogen Number Scale % (n=62) Bloodstream infections 12 19.4 Skin infections 7 11.3 Respiratory infections 26 41.9 Hepatitis 3 4.8 Tuberculosis 2 3.2 Urinary tract infections 9 14.5 Periodontitis 1 1.6 Gastroenteritis 2 3.2 Co-infection 11 17.7 Staphylococcus haemolyticus 7 11.29 Staphylococcus epidermididis 4 6.45 Staphylococcus aureus 2 3.23 Methicillin-resistant Staphylococcus aureus 4 6.45 Varicella-zoster virus 1 1.61 Herpes simplex virus 2 3.23 Coxsackie group B virus 6 9.68 Adenovirus 5 8.06 Cytomegalovirus 1 1.61 Human herpesvirus type IV (EBV) 2 3.23 Respiratory syncytial virus 2 3.23 Hepatitis B virus 2 3.23 Hepatitis C virus 1 1.61 Mycoplasma pneumoniae 6 9.68 Divergent tuberculosis bacillus 2 3.23In order to explore the related factors for concomitant systemic infections in EP, we involved the indicators of more than 90 cases in the infected and the non-infected groups into the unconditional logistic regression analysis, including demography, medical history characteristics, and some baseline laboratory investigations. For all variables included in the regression analysis, the regression model automatically excluded patients who are missing any one variable. Among the 105 EP, 10 EP patients were excluded, and 95 patients were included in the regression analysis, of which 56 were infected and 39 were not. The final results showed that joint pain and/or pustule symptoms, smoking history, hyperglycaemia, and hypoproteinaemia were related factors for EP combined with systemic infection. The results of univariate and multivariable analysis of EP combined systemic infection are shown in Table 2. The analysis method used Forward LR.
Table 2: Univariate and multivariable analysis of systemic infection in patients with erythrodermic psoriasis.
Factor Univariate analysis Multivariable analysis OR 95% CI P OR 95% CI P Gender Man 1 Woman 2.829 1.080–7.405 0.034 Age (years) <60 1 ≥60 0.720 0.329–1.577 0.412 Admission season Non-seasonal 1 Seasonal 2.58 1.023–6.506 0.045 History of smoking not 1 Yes 2.937 1.066–8.092 0.037 7.641 1.716–34.025 0.008 Arthralgia/pustules not 1 Yes 2.937 1.066–8.092 0.037 5.140 1.200–22.011 0.027 Number of comorbidities <3 1 ≥3 2.634 1.003–6.917 0.049 Conscious symptoms Light/None 1 Itching 0.689 0.284–1.674 0.411 Pain 1.063 0.309–3.659 0.923 Blood glucose Not high 1 Elevated 4.800 1.292-17.839 0.019 7.290 1.293–41.109 0.024 Hypoproteina-emia Not 1 Yes 10.543 3.996-27.817 <0.001 19.234 5.380–68.761 <0.001 Psoriasis course 0.979 0.954–1.005 0.120 Erythroderma course 1.000 0.986–1.014 0.987By comparing the data differences between the EP infected group and non-infected group, we detected inter-group differences pertaining to the following laboratory characteristics: Neutrophil Count (NEUT), C-Reactive Protein (CRP), Systemic Immune-inflammation Index (SII), Alpha-1 Acidic Glycoprotein (AAG), D-Dimer (D-D), N-Terminal pro-B-type Natriuretic Peptide (NT-ProBNP), Aspartate Transaminase (AST), Alanine Transaminase (ALT), Glutamyl Transferase (GGT), Albumin (ALB), Cholinesterase (CHE), Serum potassium (K), Serum phosphorus (P), Serum sodium (Na), Serum chloride (Cl), Serum calcium (Ca), Blood glucose (GLU), Low-Density Lipoprotein cholesterol (LDL-c), Total Cholesterol (TC). The remaining indicators were comparable, as shown in Table 3.
Table 3: Laboratory tests of blood samples of patients with erythrodermic psoriasis.
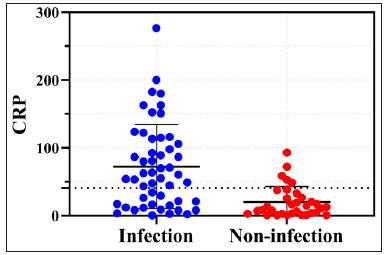
Variable Infection group Non-infected group Statistic P Neutrophil count (10^9/L) 7.6 (6.5) 4.6 (2.7) −4.657 <0.001** Lymphocyte count (10^9/L) 1.5 (1.0) 1.6 (0.8) −1.300 0.193 Platelet count (10^9/L) 293.8±116.5 276.1±87.9 0.837 0.404 C-reactive protein (mg/L) 60.6 (96.2) 12.5 (26.8) −4.511 <0.001** Systemic immune-inflammation index (10^9/L) 1340.0 (1629.8) 739.3 (501.4) −4.415 <0.001** Alpha-1 acidic glycoprotein (mg/dl) 148.0 (111.7) 121.0 (60.9) −2.142 0.032* D-Dimer (μg/mL) 2.3 (3.8) 1.1 (1.7) −2.360 0.016* N-Terminal pro-B-type natriuretic peptide (pg/mL) 614.8 (1412.0) 183.0 (470.9) −2.022 0.043* Albumin (g/L) 31.6±5.9 37.1±4.4 −5.439 <0.001** Cholinesterase (U/L) 4731.5±1988.8 6221.3±2056.5 −3.659 <0.001** Aspartate transaminase normal 45 (73.8) 36 (90.0) 4.007 0.045* abnormal 16 (26.2) 4 (10.0) Alanine transaminase normal 47 (77.0) 38 (92.7) 4.315 0.038* abnormal 14 (23.0) 3 (7.3) Glutamyl transferase normal 47 (77.0) 38 (95.0) 5.839 0.016* abnormal 14 (23.0) 2 (5.0) Serum potassium normal 47 (75.8) 39 (95.1) 6.682 0.010* abnormal 15 (24.2) 2 (4.9) Serum sodium normal 42 (67.7) 39 (95.1) 11.015 0.001** abnormal 20 (32.3) 2 (4.9) Serum chloride normal 41 (66.1) 41 (100.0) 17.444 <0.001** abnormal 21 (33.9) 0 (0.0) Serum calcium normal 27 (43.5) 28 (68.3) 6.072 0.014* abnormal 35 (56.5) 13 (31.7) Serum phosphorus normal 45 (73.8) 37 (90.2) 4.221 0.040* abnormal 16 (26.2) 4 (9.8) Low density lipoprotein cholesterol normal 32a (62.7) 29a (93.5) 10.501 0.001** elevated 4a (7.8) 2a (6.5) lower 15b (29.4) 0b (0.0) Total Cholesterol normal 37a (67.3) 31a (91.2) 6.881 0.009* elevated 2a,b (3.6) 1a,b (2.9) lower 16b (29.1) 2b (5.9) Blood glucose not elevated 40 (71.4) 36 (92.3) 6.264 0.012* elevated 16 (28.6) 3 (7.7)The ROC curve was used to verify the diagnostic performance of some laboratory investigations to indicate infection in EP. The Jordon index (sensitivity + specificity -1) corresponding to each cut-off value was calculated and the point with the largest Jordon index was considered the best cut-off value.
Scatter plots, the ROC curve, and its parameters including CRP, SII, AAG, NT-ProBNP, ALB, CHE, and TC are shown in Figures 1–2. Table 4 shows the comparison of P-values before and after adjusting for age, EP course, and body mass index using covariance for each biomarker. Through the accuracy test of the single index for the diagnosis of EP infection, we found that the comprehensive diagnostic values of CRP, SII, and ALB were high and the AUCs were greater than 0.75. TC has high specificity for diagnosing infection. The four indexes are included in multivariate regression analysis to obtain its joint predictive value and its diagnostic performance is tested.. As shown by the red curve in Figure 1, the AUC for the diagnosis of EP infection with the joint predictive value is 0.903 which is greater than any single indicator and the best cut-off value is 0.6895 with sensitivity of 79.6% and specificity of 96.15%. This regression predictive value is a relatively ideal diagnostic index, but more sample verification is needed.
Export to PPT

Export to PPT

Export to PPT

Export to PPT

Export to PPT

Export to PPT

Export to PPT

Export to PPT

Export to PPT
Table 4: P-values before and after adjusted using covariance for each biomarker
Indicators P P’ CRP <0.001 <0.001 SII <0.001 0.003 TC 0.027 0.041 CHE <0.001 0.005 ALB <0.001 <0.001 NT-proBNP 0.043 0.830 AAG 0.032 0.294 The regression predictive value <0.001 <0.001A total of 105 cases of EP were included in this retrospective analysis with a total infection rate of 45.3%, The infection rate of EP patients was higher in women compared to men, consistent with results of Zaredar N and Yiu ZZN.3,4
Although previous studies have shown that EP disease itself leads to an increase in inflammatory markers such as CRP,5,6 our study found that their rise indicates EP is complicated with infection, and new best cut-off values for CRP were obtained. We also found that SII, ALB, CHE, TC, AAG, and NT ProBNP may indicate concomitant infection in EP patients, and we explored their comprehensive diagnostic values, sensitivity, specificity, and best cut-off values. Notably, the regression predictive values of CRP, SII, ALB, and TC may be better indicators of infection in EP. However, we conducted a preliminary study with a small to medium-sized sample size, and further verification is needed in the future.
Comments (0)