Myocardial fibrosis is a crucial issue in various cardiac conditions, including chronic heart failure, valvular heart disease, diabetic cardiomyopathy, atrial fibrillation, and sudden cardiac death.[1-4] It is frequently considered a predominant pathological alteration in most myocardial diseases and a significant factor in ventricular remodeling.[3] Late gadolinium enhancement cardiac magnetic resonance (CMR) is effective in detecting focal or replacement myocardial fibrosis, but its limitation lies in the inability to determine diffuse myocardial fibrosis. In contrast, myocardial extracellular volume (ECV), measured through CMR, provides a valuable alternative for diffuse myocardial fibrosis.[5,6] Previous studies have emphasized the significance of myocardial ECV and recommended its consideration in the diagnostic evaluation of heart failure and its strong association with adverse cardiac outcomes in various myocardial diseases.[5-7]
CMR imaging has its constraints, such as longer acquisition times, limited imaging sections, and contraindications, including claustrophobia or implantable electronic devices, despite being the reference standard for noninvasive myocardial fibrosis assessment. Contrastingly, computed tomography (CT) has become widely used in cardiovascular imaging, particularly in coronary CT angiography (CCTA), providing detailed coronary anatomy and plaque characteristics for accurate risk stratification and therapy monitoring.[8] In addition, cardiac CT has been routinely used in pre-operative planning and post-operative follow-up of patients undergoing transcatheter aortic valve implantation (TAVI).[9] Recent advances in cardiac CT allowed ECV acquisition from late iodine-enhanced (LIE) scans after CCTA, especially in patients already undergoing CT for other indications, thereby bypassing some CMR limitations.[10-12]
Initially, an energy-integrating detector-based CT single-energy method was introduced for myocardial ECV quantification, requiring unenhanced images followed by a LIE scan.[13-15] Subsequently, dual-energy technology appeared, enabling iodine-based CT map creation for precise ECV quantification.[16-18] However, the adoption of dual-energy CT into guidelines and clinical practice has been constrained despite its potential, possibly due to the compromised temporal resolution and increased radiation related to dual-energy acquisitions on the energy-integrating detector CT platform.
In contrast, compared to energy-integrating detector CT, photon-counting detector (PCD)-CT maintains optimal temporal resolution for spectral acquisitions while providing improved spatial and contrast resolution, enhanced image quality, and comprehensive spectral data with each acquisition.[19-22] These advancements in cardiac imaging potentially reduced radiation exposure, minimized beam hardening and metal artifacts, increased CCTA specificity in coronary artery disease diagnosis, and improved detection of myocardial tissue characteristics and ischemia.[23-26]
Some initial studies reported on ECV quantification using PCD-CT, but standard imaging protocols remain unknown, and the influence of radiation dose and spectral image settings is still unclear.
The present study aimed to investigate the accuracy of ECV quantification using prototype PCD-CT and evaluate the influence of radiation dose and spectral image settings.
MATERIAL AND METHODSThis was a phantom study, and thus, ethical approval was not required.
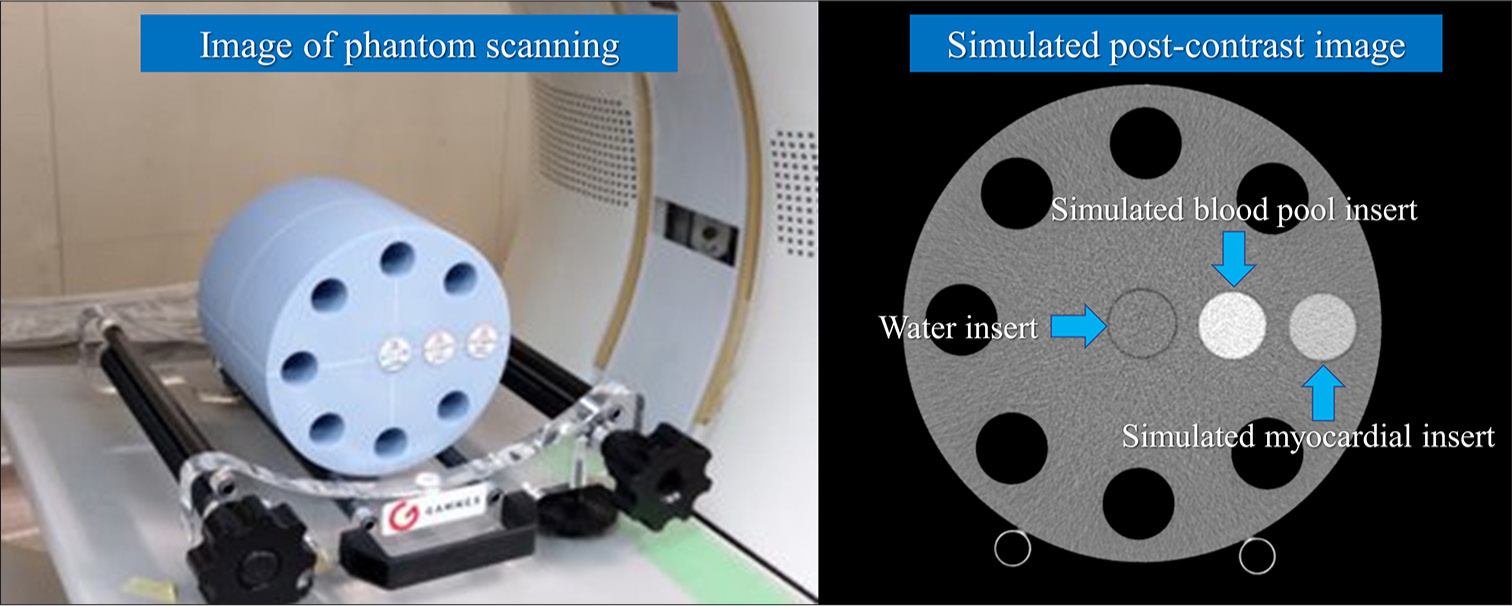
PhantomThe central portion of a multi-energy CT phantom (Sun Nuclear Corporation, Middleton, WI, USA) was utilized in our phantom study. The phantom was circular with length, height, and width of 20 cm, 20 cm (in-plane dimension), and 16.5 cm (z-direction), respectively.[27] Two types of solid cylindrical inserts for soft-tissue materials were prepared to mimic the blood pool and myocardium on pre-contrast-enhanced CT. Moreover, two types of soft tissues, including iodinated contrast materials (2 and 4 mgI/mL), were prepared to mimic blood pool and myocardial iodine enhancement on postcontrast-enhanced CT. The water material was inserted to measure the image noise.
First, the solid cylindrical inserts corresponding to the water, blood, and soft tissue were inserted into the phantom, and CT images were obtained through pre-contrast-enhanced CT. Then, the last two inserts were changed to blood with iodine and soft tissue with iodine, and CT images were obtained through postcontrast-enhanced CT [Figure 1].
Export to PPT
PCD-CT scanningThe conventional axial scan mode was used for CT scans on a prototype PCD-CT (FUJIFILM Corporation, Tokyo, Japan). The scanner parameters for the PCD-CT scan were as follows: Tube voltage of 120 kVp; tube current-time product of 105 mAs, 150 mAs, and 300 mAs; detector configuration of 16 × 0.625mm; gantry rotation time, 0.35, 0.5, and 1.0 s; slice thickness of 0.625 mm; slice interval of 0.625 mm; display field of view of 220 mm × 220 mm; and matrix size of 512 × 512.
First, two types of soft tissues were inserted into the phantom and scanned using the said conditions, assuming pre-contrast CT. Next, two types of soft tissues, including iodine contrast materials, were inserted into the same positions as pre-contrast CT and scanned using the same conditions, assuming postcontrast-enhanced CT. Simulated pre-contrast CT and post-contrast CT scans were performed thrice each. Virtual monoenergetic images (VMIs) were reconstructed at 50–100 keV with 10-keV intervals for each pre-contrast and post-contrast CT. Table 1 summarizes the acquisition parameters for PCD-CT.
Table 1: Acquisition parameters for PCD-CT.
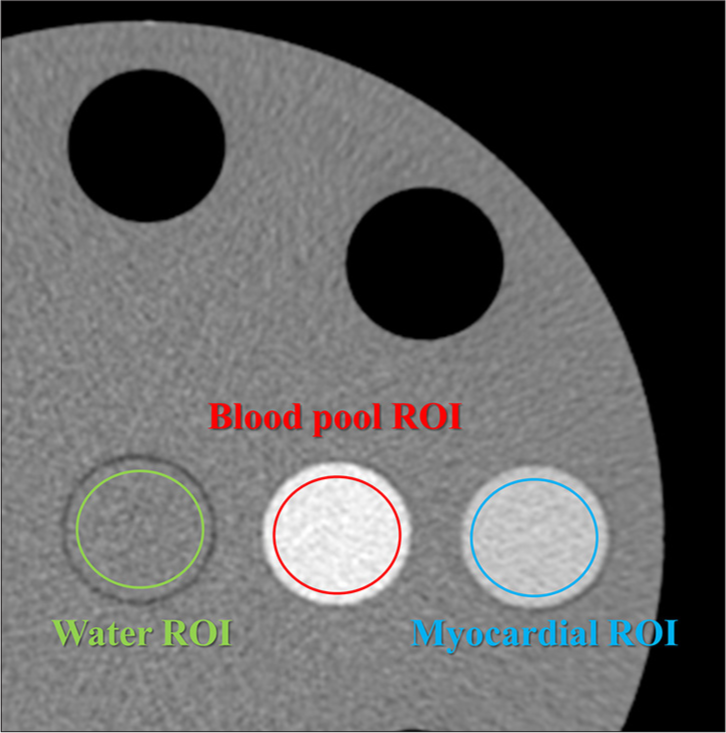
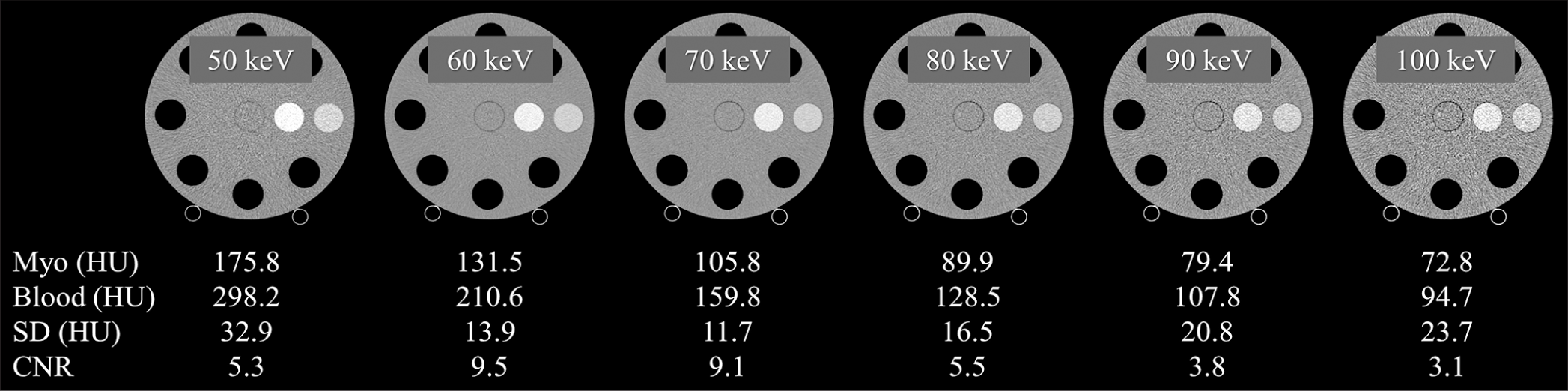
Platform 64 row-MDCT of FUJIFILM healthcare corporation Field of view 220 mm (matrix size 512×512) Gantry rotation 0.35, 0.5, and 1.0 s Tube voltage 120 kVp Tube current 300 mA (105 mAs, 150 mAs, and 300 mAs) Focal spot 1.2×1.4 mm Detector mode Multi-energy discrimination mode Energy bins 4 Z-cover in iso-center 10 mm Number of scans 3 Number of reconstructed images 16 images/scan (slice thickness of 0.625 mm) Reconstruction images 50–100 with 10-keV intervals Reconstruction filter Abdominal standard filter ECV quantificationQuantitative analysis of CT number and image noise was performed. A 15 mm diameter region of interest (ROI) was used to measure the CT numbers for two types of soft-tissue-simulated blood pool and myocardium, and the mean value of 16 images/scan and 3 scans along the z-axis was calculated for each keV image of VMI. In addition, the same ROI and the same number of images were used to determine the CT number for two types of iodinated soft-tissue-simulated blood pool and myocardial iodine enhancement. The central portion of the water insert in the phantom images was used to calculate the standard deviation (SD) of the image pixel values. Each ROI was calculated with the mean value from 16 images/scan and 3 scans along the z-axis for each keV image of VMI [Figures 2 and 3].
Export to PPT
Export to PPT
The CT-derived ECV value in the clinical situation was calculated for the global midventricular wall and each segment using the equation:
CT-derived ECV = (1 – hematocrit) × (ΔMyo/ΔBlood), where ΔMyo is myocardial Hounsfield unit (HU) (post-contrast – pre-contrast) and ΔBlood is blood pool HU (post-contrast – pre-contrast).
Our phantom study calculated the CT-derived ECV value based on the equation:
CT-derived ECV = (1 – hematocrit) × (postMyo – preMyo)/(postBlood – preBlood), where postMyo and preMyo are simulated myocardium HU in postcontrast and pre-contrast CT, whereas postBlood and preBlood are simulated blood HU in postcontrast and pre-contrast CT, respectively, with a hematocrit of 0.5. The theoretical reference ECV value is 0.25 (25%) under these conditions.
The mean ECV value was calculated using the ECVs of 16 images/scan and three scans along the z-axis at each keV setting.
The contrast-to-noise ratios in each keV image of VMI were also calculated based on the CT values of water and myocardium in the postcontrast images.
Statistical analysisAll numeric values are presented as means ± SD unless otherwise stated. Data were obtained for the mean ECV values, measured ECV SD, and the measured and reference ECV residual values at each keV setting. One-way analysis of variance was used for multiple comparisons of quantitative values with the Bonferroni post hoc test for significance between paired groups. P < 0.05 was considered statistically significant. BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan) and MedCalc (MedCalc Software, Mariakerke, Belgium) were used for all statistical analyses.
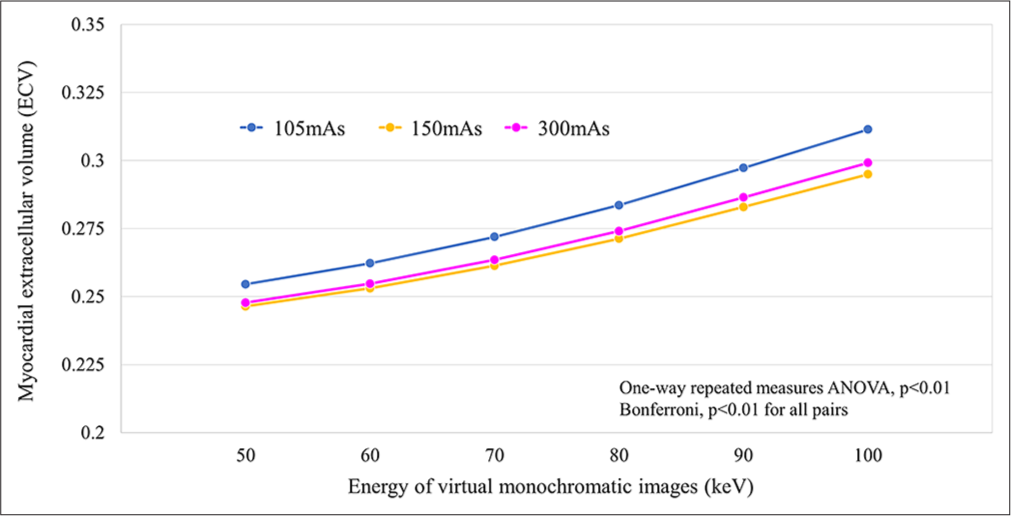
RESULTS Measured ECV valuesWe observed a consistent pattern of small yet statistically significant differences across all radiation dose settings and keV levels. Particularly, ECV values exhibited an overestimation trend at higher keV levels, regardless of the radiation dose used [Figure 4].
Export to PPT
Our study revealed a significant difference in the variance of ECV values across different keV levels, regardless of the radiation dose configuration. Notably, higher keV levels were related to greater SD in ECV measurements. This variance was most pronounced in the low-dose setting (105 mAs), indicating a heightened variability level under such conditions [Figure 5].
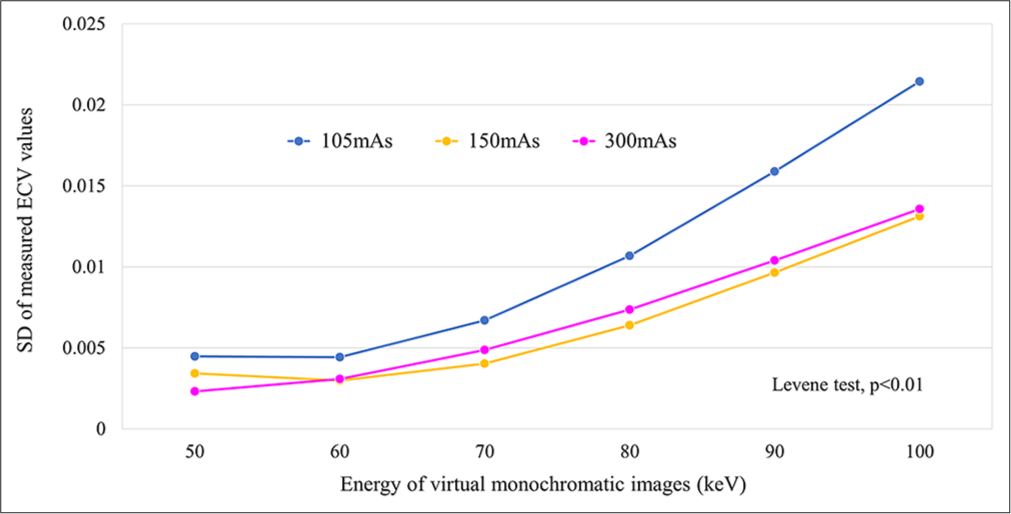
Export to PPT
Residual value for ECV measurementWe observed significantly larger residual values at higher keV levels across all radiation dose settings. Specifically, average residual values at 105-, 150-, and 300-mAs settings were 0.5– 6.1%, 0.4–4.5%, and 0.3–4.9%, respectively. Note worthily, residual values were comparatively smaller at 50- and 60-keV levels in 150- and 300-mAs settings, with no discernible significant difference [Figure 6].
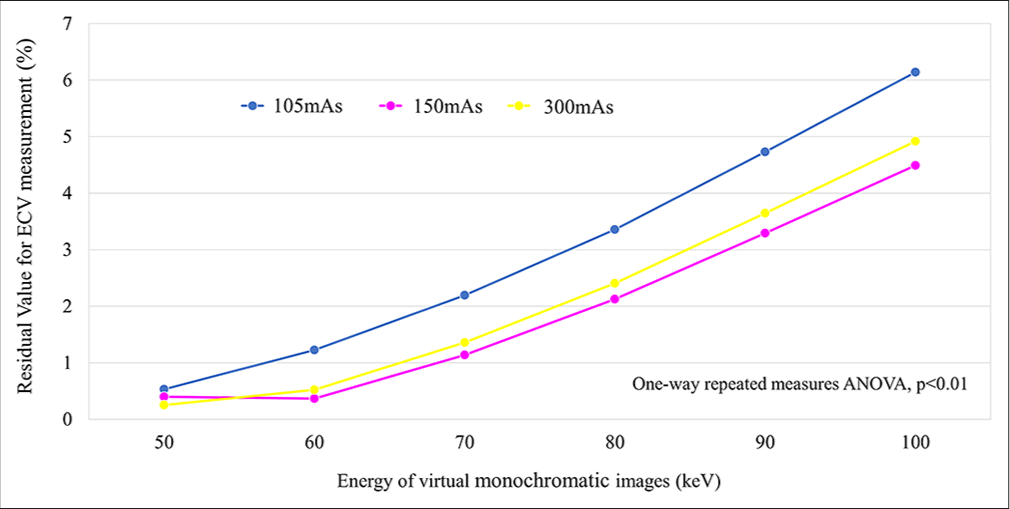
Export to PPT
DISCUSSIONOur investigation of phantom studies using PCD-CT generated distinctive results regarding ECV measurements. We consistently observed small yet statistically significant differences across all radiation dose settings and keV levels, with a possible ECV value overestimation at higher keV levels, regardless of the radiation dose used. In addition, we determined a substantial variance in ECV values across different keV levels, regardless of the radiation dose configuration, with higher keV levels demonstrating greater SD, particularly pronounced at the low-dose setting (105 mAs). Furthermore, we observed significantly greater residual values at higher keV levels across all radiation dose settings. These residuals may be improved by refining the use of the energy information derived from the system. On the other hand, the 150- and 300-mAs settings exhibited smaller residual values at 50- and 60-keV levels, allowing accurate ECV quantification.
The image quality of VMI, that is, contrast and image noise, generated by this PCD-CT scanner may have affected the accuracy of ECV quantification. In particular, contrast may be a major factor in ECV quantification errors. Specifically, lower contrast was observed at higher keV settings (≥70 keV) compared to 50 keV and 60 keV settings. At lower contrast at higher keV settings, even small CT value errors may have a large contribution to ECV accuracy degradation. As an additional factor, increased image noise at lower radiation dose settings could also contribute to the diminished ECV quantification accuracy. These combined factors may have contributed to the error in measuring the difference in CT values between the simulated myocardial tissue and the blood pool and then calculating the ECV. Image quality improvements are anticipated in PCD-CT with ongoing CT technology advancements. For instance, the integration of deep learning-based CT reconstruction techniques,[28] currently used in energy-integrating detector-based CT, will be implemented in PCD-CT scanners in the near future, thereby substantially improving image quality.
This study evaluated the ECV based on CT numbers. However, ECV quantification in PCD-CT based on iodine density is achievable through spectral analysis. Our future aim is to validate ECV quantification based on iodine density using PCD-CT. Utilizing analysis grounded on iodine density can improve the precision of ECV quantification with PCD-CT.
Mergen et al. conducted a study to investigate the feasibility and accuracy of quantifying ECV using dual-source PCDCT (NAEOTOM Alpha; Siemens Healthineers).[29] They included 30 patients with severe aortic stenosis undergoing pre-procedural workup for TAVI. The study used dual-energy iodine density-based ECV quantification and concluded that dual-source PCD-CT enabled sufficient ECV quantification at a low radiation dose. However, they did not compare ECV results with those obtained via CMR, which is considered the reference standard modality; thus, they could not definitively comment on the accuracy of PCD-CT in ECV quantification. Moreover, they should have thoroughly investigated imaging and image reconstruction parameter optimization.
Aquino et al. conducted a study comparing ECV quantification using a dual-source PCD-CT (NAEOTOM Alpha; Siemens Healthineers) with CMR.[26] They revealed strong correlations between ECV measurements obtained from dual-energy (iodine-based) and single-energy (CT number-based) PCD-CT scans (r = 0.91). Dual-energy PCDCT demonstrated a 40% lower radiation dose compared to single-energy PCD-CT. Dual-energy PCD-CT demonstrated strong correlations when compared to CMR (r = 0.91) but with a slight ECV overestimation (approximately 2%). Single-energy PCD-CT exhibited a similar association with CMR but slightly underestimated ECV (by 3%). However, they did not evaluate the imaging techniques, image reconstruction methods, and image quality of PCD-CT. Optimizing the imaging parameters is crucial for the clinical implementation of ECV quantification using PCD-CT.
The present study has some limitations. First, our phantom study did not account for cardiac motion, which could potentially affect both image quality and ECV quantification accuracy during clinical imaging of patients. In addition, factors, such as patient dimensions and artifacts originating from ribs and vertebrae, may influence ECV quantification. Thus, further validation through clinical studies is necessary. Second, our study utilized only the conventional standard image reconstruction filter. In recent years, advancements in deep-learning-based image reconstruction methods have emerged, and applying these innovative techniques to PCD-CT may further enhance the accuracy of ECV quantification. Third, our study solely focused on evaluating ECV quantification using CT numbers and did not validate methods reliant on iodine density. Conducting foundational research is important before considering the adoption of ECV quantification derived from iodine density to ascertain the accuracy of iodine density quantification through PCD-CT. Finally, PCD-CT scanners from other vendors use different detector systems, potentially causing variations from the outcomes obtained with our prototype PCD-CT scanner.
CONCLUSIONEstablishing suitable keV and radiation dose parameters is imperative when quantifying ECV with PCD-CT, as varying keV levels could cause disparities in quantification values and measurement fluctuations. This prototype PCD-CT scanner facilitated precise ECV quantification at 50 and 60 keV with 150- and 300 mAs settings.
Comments (0)