Congenital portosystemic shunt (CPSS) is a rare vascular anomaly in which portal blood bypasses the liver and flows directly into the systemic circulation, with an incidence estimated at 1 in 30,000–50,000 live births.[1] CPSS arises from abnormal vascular development during the 4th–6th weeks of embryogenesis, likely due to remnants of fetal circulation or other developmental anomalies.[2] Common clinical manifestations include hyperammonemia, neonatal cholestasis, liver dysfunction, hypoglycemia, thrombocytopenia, and coagulation disorders.[3] In the long term, complications such as portopulmonary hypertension and hepatic tumors, including nodular hyperplasia, have been reported.[4] Diagnosis is primarily based on imaging studies, with intrahepatic CPSS sometimes undergoing spontaneous closure within the first 2 years of life, while extrahepatic CPSS typically persists and requires therapeutic intervention.[2,5] Treatment options depend on the type of shunt, patient age, and severity of symptoms.[6,7] In cases where spontaneous closure of intrahepatic CPSS is anticipated, conservative management is pursued. Interventional treatments include transcatheter shunt embolization,[6,7] surgical ligation of the shunt,[8] and, in severe cases, liver transplantation may be considered.[9] Early diagnosis and appropriate therapeutic interventions can lead to favorable outcomes in many cases.[5] We report the case of an 11-year-old patient with CPSS who was managed with long-term follow-up. During this period, we observed a shift in shunt blood flow dominance from the celiac artery (CA)-splenic vein (SPV) system to the superior mesenteric artery (SMA)-superior mesenteric vein (SMV) system as the patient grew. Following transcatheter shunt embolization, shunt closure and improvement in laboratory values were confirmed. We discuss the timing and choice of treatment considering the present case.
CASE REPORTThe patient was an 11-year-old male. There were no abnormalities during his mother’s pregnancy, and he was born through spontaneous vaginal delivery at 39 weeks and 3 days of gestation, with an appearance, pulse, grimace, activity and respiration (APGAR) score of 9 at 1 min after birth. His birth weight was 3263 g. Although he demonstrated good feeding and weight gain, neonatal mass screening revealed elevated blood galactose levels (10 mg/dL) on days 5 and 16 after birth (cutoff value: <8 mg/dL). At 24 days old, he was admitted for the evaluation of possible causes of hypergalactosemia. Upon admission, his weight was 4396 g, and his height was 56 cm. No heart murmurs or hepatosplenomegaly were detected, and his abdomen was soft without palpable masses. He had normal male external genitalia with no apparent neurological abnormalities. Blood tests showed liver enzyme levels with aspartate aminotransferase at 28 IU/L, alanine aminotransferase at 18 IU/L, gamma-glutamyl transpeptidase at 64 IU/L, alkaline phosphatase at 1,347 IU/L, and lactate dehydrogenase at 277 IU/L. Total bilirubin was elevated at 5.4 mg/dL, with direct bilirubin at 0.7 mg/dL. The ammonia level (NH3) was elevated at 112 μg/dL. Coagulation studies showed a prothrombin time–international normalized ratio of 1.01 and an activated partial thromboplastin time of 36.1 s. Fibrin degradation products were 2.1 μg/mL, fibrinogen was 272 mg/dL, and antithrombin III was 75%, indicating a normal coagulation profile. Total bile acids (TBA) were elevated at 74.4 μmol/L.
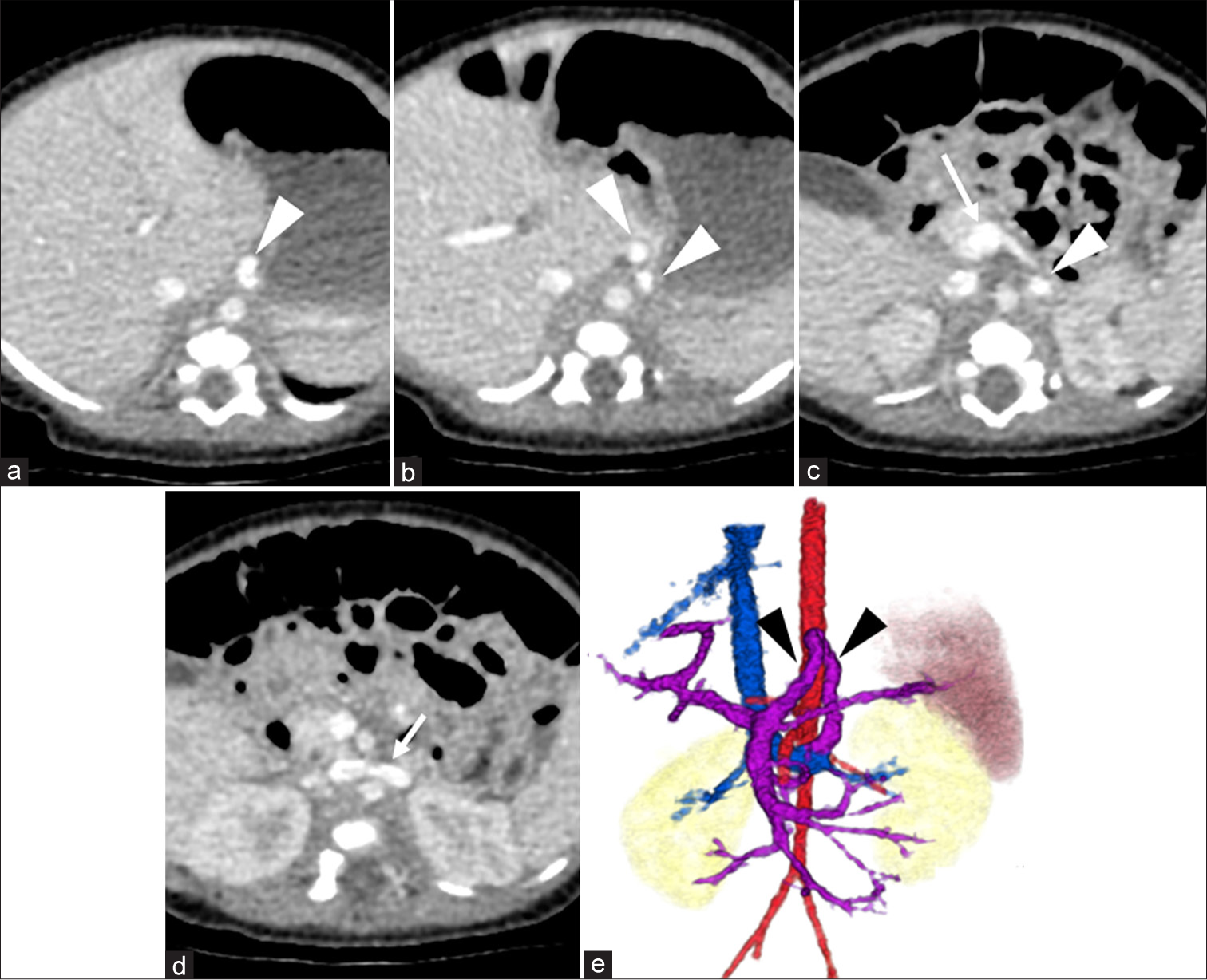
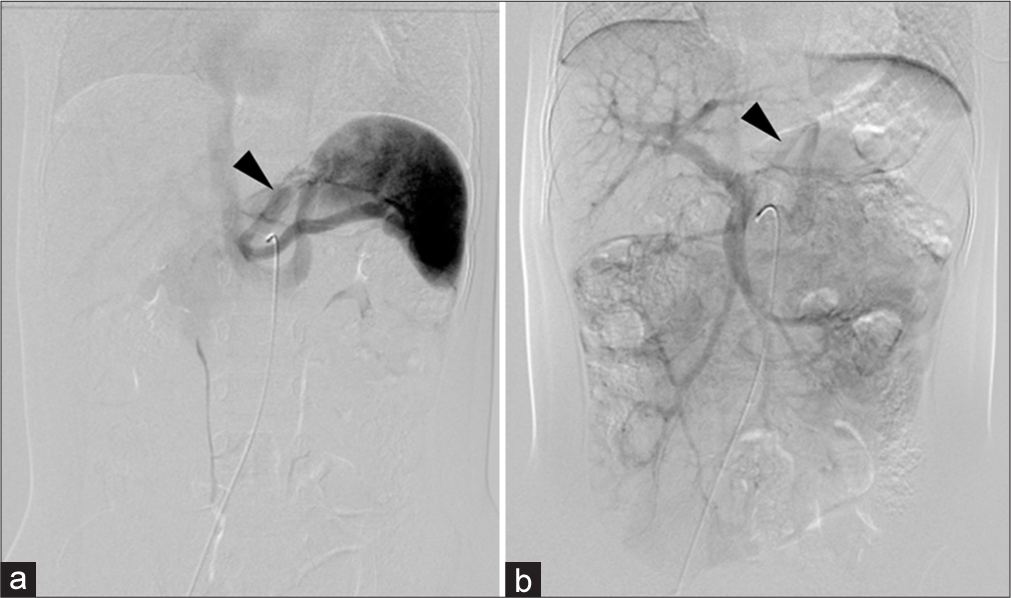
Abdominal ultrasound suggested a fetal circulation anomaly and dynamic contrast-enhanced computed tomography (CT) revealed a shunt between the portal vein and left renal vein, diagnosing CPSS [Figure 1]. The long axis diameters of the portal vein and spleen were 4.4 mm and 47.9 mm, respectively. The patient was managed with lactose-free formula and advised to avoid high-galactose and high-protein foods, resulting in satisfactory growth and development. While NH3 and TBA remained slightly elevated, overall growth was normal. At 2.5 years, despite no symptoms of hyperammonemia and no evidence of cataracts on ophthalmologic examination, the contrast-enhanced CT and angiography were performed to evaluate the CPSS and potential closure options due to long-term risks to brain, heart, and lung function. The CT revealed that the long-axis diameters of the portal vein and spleen were 4.9 and 61.1 mm, respectively. Angiography from the superior mesenteric artery (SMA) and celiac artery (CA) confirmed a shunt from the portal vein to the left gastric vein and left renal vein [Figure 2]. Balloon catheterization measured portal pressure at 10 mmHg during both occlusion and release. At that time, the family chose to postpone treatment, and the patient was observed.
Export to PPT
Export to PPT
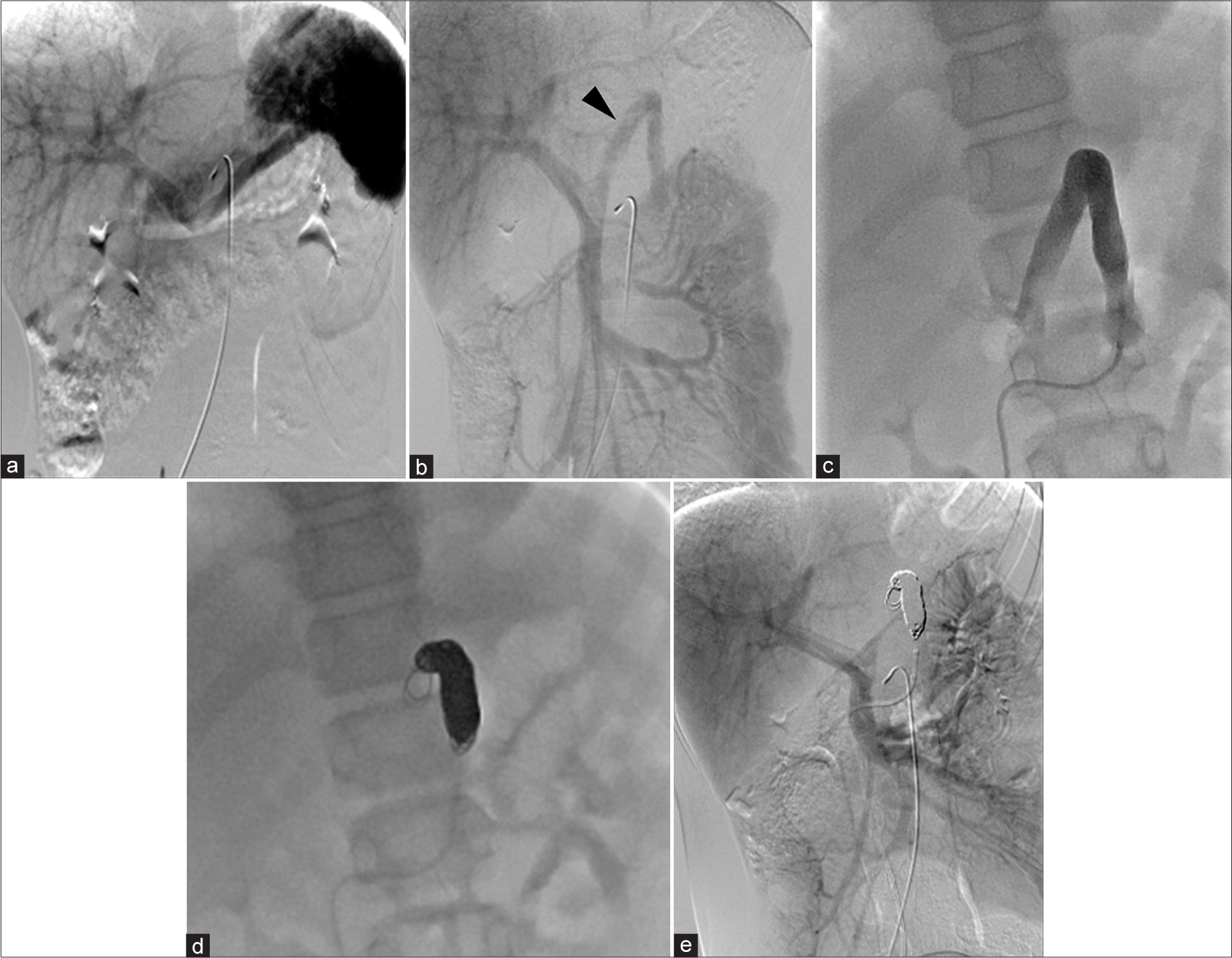
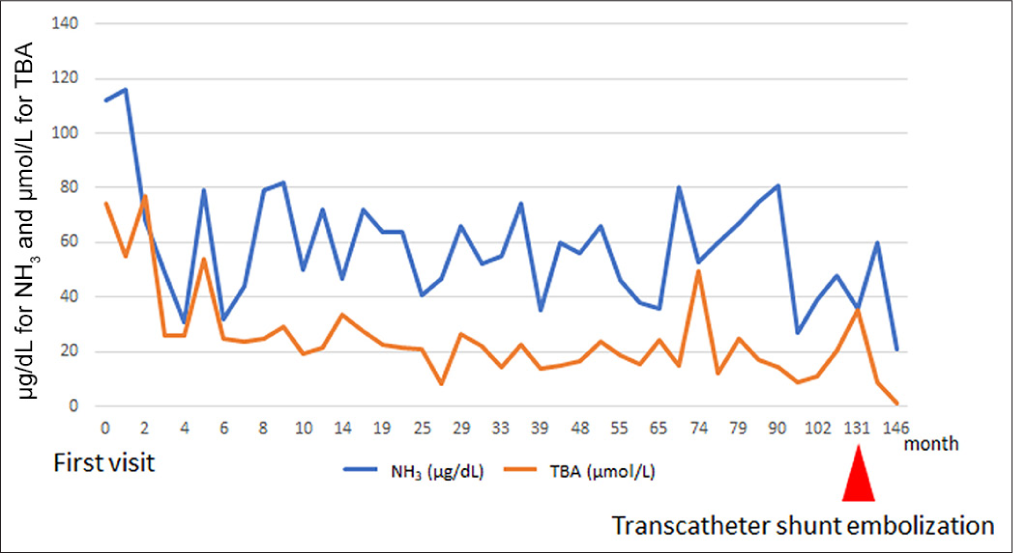
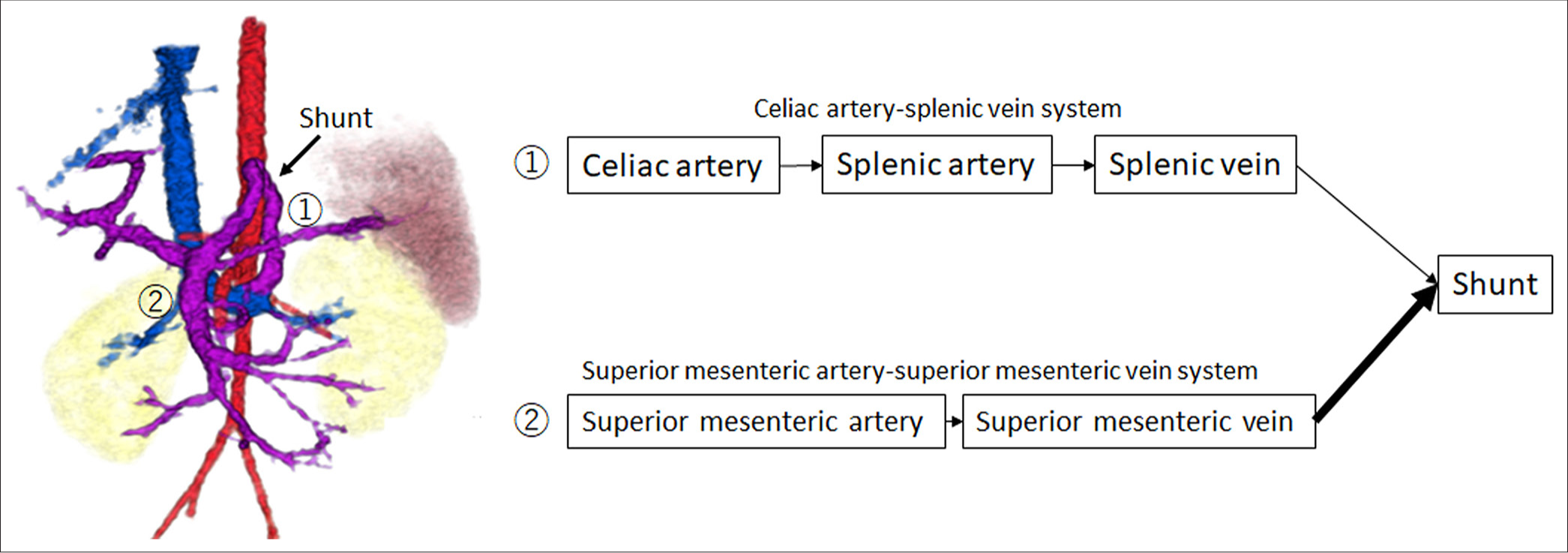
CT at age 6 showed an increase in organ size with growth but no significant change in the CPSS diameter (6 mm). There were no findings suggestive of right ventricular overload or abnormal dilation of the pulmonary artery. In addition, brain magnetic resonance imaging (MRI) performed during the same period showed no clear signal changes indicative of hyperammonemia. The patient and his family were lost to follow-up due to family circumstances and school activities. At age 11, he and his family became concerned about his CPSS condition and returned to the hospital. He was admitted for CT, angiography, and possible embolization. The CT revealed that the long-axis diameters of the portal vein and spleen were 7.6 mm and 81.2 mm, respectively, suggesting gradual enlargement consistent with normal development compared to ages 2.5 and 6. Angiography from the SMA and CA confirmed the shunt from the portal vein to the left gastric and left renal veins. Blood flow of the shunt from the superior mesenteric artery (SMA)-superior mesenteric vein (SMV) system was more prominent than that from the celiac artery (CA)-splenic vein (SPV) system, indicating a shift in dominance to the SMV [Figure 3]. The portal pressure measured in the shunt vessel was 6 mmHg during balloon occlusion and release, showing no significant pressure gradient upon balloon closure. In this process, the adequacy of balloon occlusion was confirmed through contrast injection from the balloon tip. The gradient of the portal pressure was zero between balloon occlusion and release. With careful attention to preserving the normal left gastric and left renal veins, a total of 12 coils were used to embolize the shunt vessel, beginning from the top of its inverted U-shape. Post-embolization angiography from the SMA and CA confirmed the absence of shunt blood flow, completing the procedure. Following embolization, NH3 and TBA levels decreased [Figure 4], and the patient has remained asymptomatic.
Export to PPT
Export to PPT
DISCUSSIONIn cases of CPSS, intrahepatic shunts may close spontaneously within the first 2 years of life, whereas large shunts or extrahepatic shunts generally persist throughout life and often require therapeutic intervention.[2,5,10,11] Regarding the timing of treatment, it is recommended to promptly close shunts that persist beyond the 1st year without waiting for the onset of complications.[10] However, the optimal timing and criteria for intervention in asymptomatic CPSS cases remain controversial, as no standardized guidelines have been established.[12] In this case, the shunt’s size and blood flow were evaluated by dynamic contrast-enhanced CT and angiography; however, treatment was delayed until the patient was 11 years old owing to personal family-related factors. Although treatment was delayed due to personal family-related factors, the observation of shifting hemodynamics over time was educational, leading us to present this case. During this period, we observed a shift in shunt blood flow from the CA-SPV system to the SMA-SMV system, which supported the decision to proceed with shunt embolization. When shunt blood flow becomes predominantly SMA-SMV dependent, it means that a larger portion of the nutrient-rich mesenteric blood flow is bypassing the liver [Figure 5]. This is particularly problematic because the mesenteric venous blood contains higher concentrations of intestinal toxins and nitrogenous compounds like ammonia that normally should undergo first-pass metabolism in the liver.[13] In this case, no evidence of right ventricular overload or signal abnormalities on brain MRI was observed; however, a limitation of this case is that hyperammonemia may have caused changes that are not detectable on imaging.
Export to PPT
There are ongoing concerns regarding the risk of coil migration when placing metal coils through transcatheter methods in pediatric patients. For adults, the reported rate of coil migration ranges from 0.3% to 6%.[14] Cases of coil migration have been reported more than a year after placement, with a median occurrence of 2.3 years (range, 1.2–12 years).[15] In pediatric patients, changes in vascular structure with growth could potentially lead to discrepancies between the coil and vessel diameter, though data on the incidence of migration in pediatric cases have not been reported to our knowledge. In this case, we plan to monitor coil placement with annual abdominal X-rays. In addition, the compatibility of magnatic resonance imaging (MRI) following coil placement raises concerns over the long term. All coils used in this case were confirmed compatible with MRI up to 3T, enabling future MRI imaging if required for other conditions. In contrast to transcatheter shunt embolization, surgical ligation of the shunt offers several advantages. Abdominal X-rays are generally not required following the procedure. In addition, surgical ligation avoids the need to consider MRI compatibility issues associated with metal implants.
Treatment options for CPSS typically include surgical ligation of the shunt or transcatheter shunt embolization. In cases with portal pressures exceeding 32 mmHg, a staged closure approach may be considered. In this case, there was no significant pressure gradient between occlusion and release, with a low portal pressure of 6 mmHg during balloon occlusion. Given that we expected no rise in portal pressure from coil embolization, we proceeded with a one-stage coil embolization. Comparison of SMA and CA angiograms at 2.5 years (first angiography) and 11 years (second angiography) showed that shunt flow had become increasingly SMA-SMV system dominant in the second angiography. This shift indicated an increased systemic circulation of the nutrient-rich mesenteric blood through the shunt. These findings suggest that earlier intervention may have been more suitable if consent from the family had been obtained. In pediatric cases, however, the decision-making of the patient and their family must be respected. Although transcatheter coil embolization had radiation exposure risks compared to surgery, the patient’s total exposure during angiography (175.6 mGy and 4,099.4 μGy•m2) was within acceptable levels when compared to diagnostic reference levels.[16]
When choosing between transcatheter embolization and surgery, the shunt vessel’s shape and position are critical factors. In this case, the shunt had a straightforward pathway from the portal vein to the left gastric and left renal veins, making it accessible through the femoral vein. When the shunt anatomy is complex, involves multiple vessels, or is difficult to access with a catheter, surgery may be preferable.[8] In addition, larger vessels and longer shunt lengths require more coils for effective embolization. In this case, earlier intervention at a smaller shunt diameter might have reduced the number of coils needed; however, careful consideration of timing is essential, taking into account family preferences and the risk of coil migration.
CONCLUSIONThis case demonstrated a gradual shift in shunt blood flow from the CA-SPV system to the SMA-SMV system as the patient grew, supporting the indication of shunt embolization. Further research is needed to determine the optimal timing for shunt embolization in pediatric CPSS cases.
Comments (0)