The established link between chronic inflammation and cancer has illuminated the intricate biological processes that fuel carcinogenesis. Chronic inflammation, which can be driven by persistent infections, autoimmune disorders, or prolonged exposure to environmental carcinogens such as tobacco smoke, industrial pollutants, or asbestos, sets the stage for continuous tissue damage and abnormal cellular proliferation. This sustained inflammatory environment creates fertile ground for neoplastic transformations by promoting cellular repair mechanisms that, paradoxically, can lead to the onset of malignancies (Khandia and Munjal, 2020). Inflammation not only supports the survival and proliferation of cancer cells but also fosters metastasis (Hasegawa et al., 2006) by reshaping the tumor microenvironment (TME) to favor malignant growth (Afshari et al., 2022). Epigenetic alterations induced by inflammatory signaling further entrench the cancer-inflammation nexus, as oncogenes are activated and tumor suppressor genes are silenced through DNA methylation, histone modification, and chromatin remodeling (Tan et al., 2022). Within the TME, immune cells like macrophages, neutrophils, and T-cells play dual roles, either suppressing or facilitating tumor progression, thus complicating therapeutic interventions (Li et al., 2019; Segovia et al., 2019; Vredevoogd et al., 2019; Segovia et al., 2020).
A key mechanism by which tumors evade immune surveillance is through the upregulation of immune checkpoints such as programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), which inhibit T-cell activity and allow cancer cells to thrive unchecked (Wei et al., 2019). The successful targeting of immune checkpoints has revolutionized cancer treatment by reinvigorating the immune system’s ability to combat tumors, as evidenced by the clinical efficacy of anti-PD-1/PD-L1 and anti-CTLA-4 therapies (Jacquelot et al., 2019; Theivanthiran et al., 2020). Notably, the transcription factor NF-κB plays a pivotal role in regulating PD-L1 expression, directly binding to the promoter of the PD-L1 gene and upregulating its transcription. Additionally, NF-κB influences post-transcriptional pathways that stabilize PD-L1, contributing to tumor immune evasion (Antonangeli et al., 2020). Recent discoveries reveal that cancer stem-like cells (CSCs) exhibit heightened PD-L1 expression, further protecting them from immune attacks. The regulatory mechanisms that enrich PD-L1 expression in CSCs remain largely unexplored, underscoring a critical gap in our understanding of tumor resistance (Hsu et al., 2018).
Interestingly, the mesenchymal-epithelial transition (MET) has been identified as a novel mechanism regulating PD-L1 stability in CSCs, with studies showing that targeting this pathway can enhance the efficacy of cancer immunotherapy (Sharaf et al., 2014). The influence of chromatin remodeling in response to inflammatory signals has also gained attention, with genes like IL-1A and IL-1B becoming dynamically repositioned within transcription factories during immune responses. This spatial organization of gene expression is crucial for mediating inflammatory effects within tumors (Papantonis et al., 2012; Audia and Campbell, 2016; Horton et al., 2016; Marazzi et al., 2018; Shokri et al., 2018; Das et al., 2021; Ding et al., 2022). Similarly, the role of inflammasomes, particularly NLRP3, in cancer has been increasingly recognized, linking inflammation directly to cancer progression (Ding et al., 2022).
Despite significant advances in immunotherapies, including immune checkpoint inhibitors and CAR T-cell therapies, certain cancers, such as pancreatic and prostate cancers, have shown limited responses to these treatments (Ye et al., 2024). Understanding the molecular drivers of resistance, such as aberrant inflammatory signaling and immune evasion, remains key to improving the clinical outcomes of these malignancies. Combining immunotherapy with other treatment modalities, such as chemotherapy or radiotherapy, has shown promise, but additional research is required to fully unlock the potential of these therapeutic strategies (Latchman et al., 2001; De Marzo et al., 2007; Gandaglia et al., 2013; Powles et al., 2014). Furthermore, focusing on critical protein networks in cancer progression offers an opportunity to develop more precise, multi-targeted therapies that address the complexity of cancer’s molecular landscape. This approach holds the promise of better patient outcomes and a more profound understanding of the interplay between inflammation, the immune system, and cancer.
1.2 Comparative expression analysis of DNMT-1, Dicer1, and PD-1/PD-L1 in normal and prostate cancer patients and their correlation with gleason scoreFigure 1 provides a comprehensive analysis of the expression levels of specific genes DNMT-1, PD1, Dicer1, and PD-L1 in prostate cancer patients compared to normal individuals, sourced from https://ualcan.path.uab.edu/index.htm. These levels are correlated with a Gleason score, a grading system used to evaluate the aggressiveness of prostate cancer.
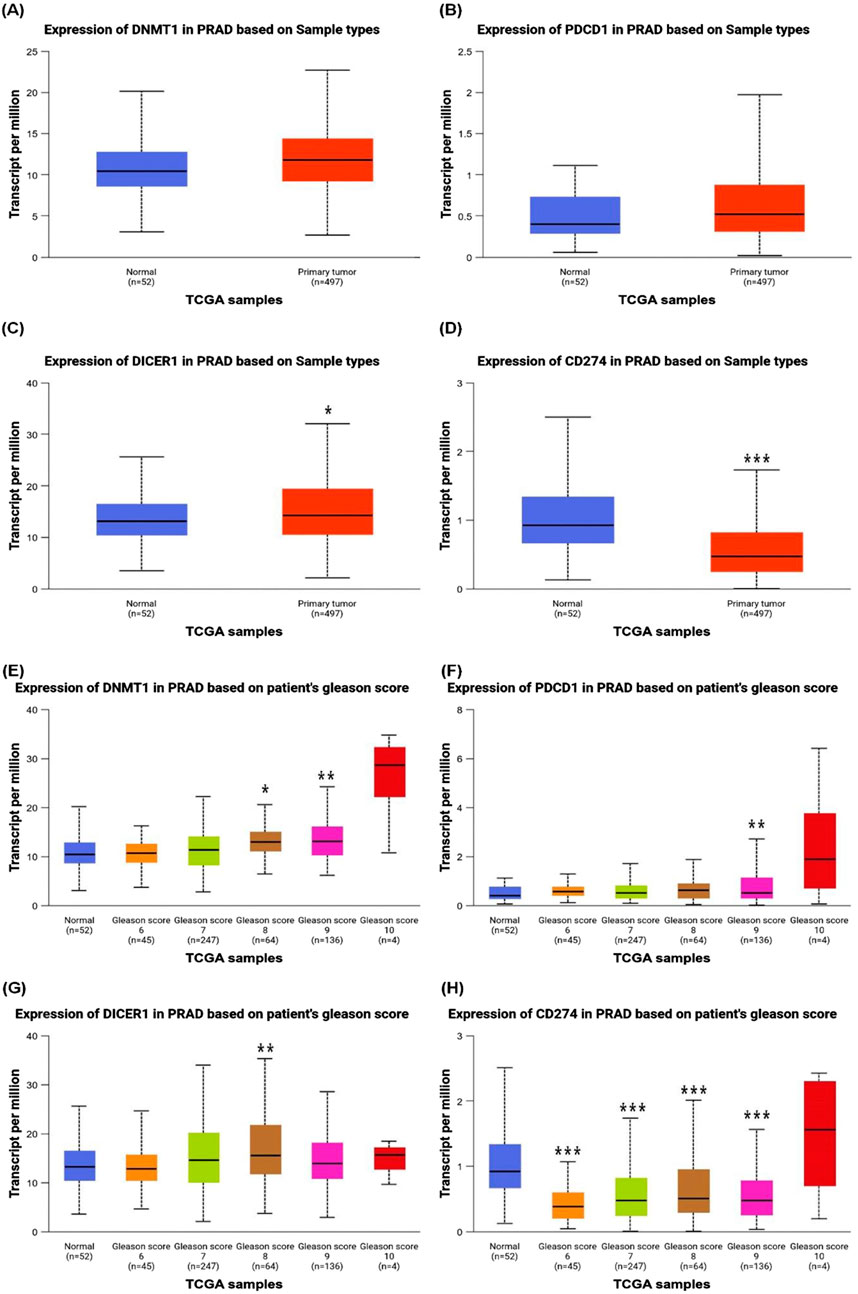
Figure 1. Differential Gene Expression Profiles in Prostate Cancer Patients. Graphical representation of the expression levels of DNMT-1, PD1, Dicer1, and PD-L1 in prostate cancer patients compared to normal individuals. The expression of (A) DNMT-1, (B) PD1, (C) Dicer1, and (D) PD-L1 is shown, highlighting significant differences between normal and cancerous tissues (p-values: p* < 0.05, p *** < 0.001, as indicated). Additionally, panels (E) DNMT-1, (F) PD1, (G) Dicer1, and (H) PD-L1 illustrate the correlation of their expression with the Gleason score, representing prostate cancer progression and aggressiveness. Statistical significance is indicated by corresponding p-values, demonstrating the relationship between molecular expression and tumor grade (p-values: p* < 0.05, p** < 0.01, p *** < 0.001, as shown).
Figure 1A reveals that DNMT-1 expression is significantly elevated in prostate cancer patients compared to normal individuals. DNMT-1, a key enzyme involved in DNA methylation, plays a crucial role in maintaining the epigenetic landscape of cancer cells, contributing to tumor progression and silencing tumor suppressor genes (Park, 2023). Figure 1B describes the expression of PD1, a protein that negatively regulates T-cell activity, that is implicated in immune evasion by tumors, and that is notably higher in cancer patients than in normal individuals. This upregulation suggests that prostate cancer cells may employ immune checkpoint mechanisms to evade immune surveillance. In silico clinical trials, while offering valuable insights, must be interpreted with an understanding of their inherent limitations (Creemers et al., 2023). In our analysis, no statistically significant correlation was observed between DNMT-1 and PD-1 expression levels and patient outcomes. These findings highlight the importance of complementing in silico results with experimental validation and clinical studies to ensure robust and reliable conclusions (Figures 1A, B).
Figure 1C shows that Dicer1, an enzyme essential for microRNA processing (Goel and Goel, 2024), also exhibits increased expression in prostate cancer tissues compared to normal tissues. Elevated Dicer1 levels may contribute to the dysregulation of microRNA pathways that are critical in cancer cell proliferation, invasion, and metastasis (Dobrijević et al., 2021).
In normal cells, PD-L1(CD274) expression is typically low or tightly regulated, and its upregulation occurs primarily in response to inflammatory signals (e.g., cytokines like IFN-γ) or under conditions of immune activation (Sun H. Y et al., 2020). As shown Figure 1D, overall downregulation of PD-L1 expression in prostate cancer tissues compared to normal tissues. This suggests that, at the transcriptional level, PD-L1 expression may not universally increase in prostate cancer.
The expression dynamics of PD-L1 (CD274) in prostate cancer are complex and can vary depending on tumor stage, tumor microenvironment, and specific cellular contexts. While some studies report elevated PD-L1 levels in advanced or metastatic prostate cancer (Zavridou et al., 2021). Figure 1H indicates a positive correlation between PD-L1 expression and higher Gleason scores. This suggests that, while PD-L1 expression may be lower overall in prostate cancer tissues, higher-grade tumors may exhibit relatively elevated PD-L1 levels compared to lower-grade tumors within the prostate cancer cohort. PD-L1 binds to PD1 on T-cells, leading to the suppression of the immune response, which allows cancer cells to thrive by avoiding immune detection and destruction (Topalian et al., 2015; Chen and Mellman, 2017).
The correlation of gene expression with Gleason score is described in Figure lG−H. Figure 1E shows a positive correlation between DNMT-1 expression and the Gleason score, indicating that DNMT-1 expression increases with the severity and aggressiveness of prostate cancer. Figure lF reveals similar trend with PD1, where its expression levels rise in tandem with the Gleason score. This suggests that as prostate cancer becomes more aggressive, it increasingly relies on immune checkpoint pathways to evade the immune response. The expression of Dicer1 also shows a positive correlation with the Gleason score, as shown in Figure lG. This indicates that more aggressive prostate cancers may depend on the dysregulation of microRNA processing pathways, mediated by Dicer1, to sustain their growth and spread. Finally, Figure lH shows that PD-L1 expression also shows a strong positive correlation with the Gleason score, reinforcing the notion that more aggressive prostate cancers are more adept at suppressing immune responses, which is crucial for their survival and proliferation.
The data presented in this figure suggest that the expression of DNMT-1, PD1, Dicer1, and PD-L1 is markedly higher in prostate cancer patients compared to normal individuals and that their expression levels are positively correlated with the Gleason score. This indicates that these genes may play a critical role in the progression and aggressiveness of prostate cancer, and they could serve as potential biomarkers for disease severity or as targets for therapeutic intervention. The strong correlation between these gene expressions and the Gleason score underscores their potential utility in predicting the aggressiveness of prostate cancer and tailoring treatment strategies accordingly.
1.3 Prostate cancer pathologyProstate cancer is the second most commonly diagnosed cancer worldwide and the fifth leading cause of cancer-related mortality among men as of 2022, with an estimated 1.5 million new cases and 397,000 deaths globally. Notably, in 52 countries, it stands as the leading cause of cancer-related deaths among men, underscoring its significant global health burden (Bray et al., 2024). Prostate cancer is a complex disease influenced by numerous biological, environmental, and genetic factors, with inflammation playing a significant role in its development and progression. The prostate is an immunocompetent organ, often the site of a small number of inflammatory cells (Gandaglia et al., 2013). Prostatic inflammation can stem from various sources, such as viral or bacterial infections, dietary factors, hormonal imbalances, autoimmune responses, and even urine reflux (De Marzo et al., 2007; Gandaglia et al., 2013). Accumulating evidence from epidemiological, histopathological, and molecular research strongly supports a link between chronic inflammation and the initiation and advancement of prostate cancer (De Nunzio et al., 2011; Sfanos et al., 2014). As inflammation persists, it creates a microenvironment that fosters cellular proliferation, DNA damage, and epigenetic changes, all of which contribute to malignant transformation and tumor progression.
A cornerstone of prostate cancer treatment, especially in cases of locally advanced or metastatic disease, has been androgen deprivation therapy (ADT), which can be administered either pharmacologically or surgically (El Badri et al., 2019). Androgen suppression has long been recognized as a vital strategy in controlling prostate cancer, dating back to 1941 when it was discovered that reducing testosterone levels could slow tumor growth (Huggins and Hodges, 1941). Historically, castration, along with the use of estrogen injections to inhibit testosterone production, proved effective in managing the disease (Mcleod, 2003; Sharifi et al., 2005). Over time, ADT evolved to offer more sophisticated approaches such as anti-androgens that block testosterone receptors on prostate cancer cells. Another widely used method involves luteinizing hormone-releasing hormone (LHRH) agonists that prevent the anterior pituitary gland from secreting luteinizing hormone, thereby reducing testosterone production (Litwin and Tan, 2017).
Emerging research has highlighted the intricate interplay between androgen signaling, growth hormone (GH), and estrogen pathways in prostate cancer. GH and gonadal systems are intimately linked in terms of growth, development, and metabolism, yet their precise regulatory interactions remain only partially understood (Marin et al., 1994). The enzyme aromatase, which converts androgens into estrogens in various tissues, has revealed a deep mechanistic connection between estrogen biology and GH regulation. Local estrogen production can exert paracrine effects that extend beyond classical endocrine pathways, influencing GH secretion even in the absence of the androgen receptor (Birzniece and Ho, 2021). Additionally, 5α-reductase, which converts testosterone into dihydrotestosterone (DHT), plays a crucial role in androgen action at the tissue level, further impacting GH regulation and prostate cancer progression (Veldhuis et al., 2009).
Testosterone acts as a prohormone, its effects being mediated through its conversion into DHT and estradiol (E2) in tissue-specific contexts (Jasuja et al., 2013; van den Beld et al., 2018; Kaufman et al., 2019). Research has demonstrated that inhibiting 5α-reductase, thereby reducing DHT, does not affect testosterone’s ability to increase GH secretion. However, inhibition of aromatase, which reduces estradiol levels, significantly disrupts GH production, revealing estradiol as a critical mediator of GH’s effects in prostate cancer (Link et al., 1986; Birzniece and Ho, 2021). In recent clinical studies, 5α-reductase inhibitors have been explored as a potential therapeutic option for prostate cancer patients (Hu et al., 2020). Finasteride and dutasteride, two synthetic 5α-reductase inhibitors (5ARIs), are commonly recommended for treating conditions such as alopecia, lower urinary tract symptoms, and benign prostatic hyperplasia. However, these medications carry a range of significant side effects, including a heightened risk of high-grade prostate cancer as well as neurological, psychiatric, endocrine, metabolic, and ophthalmological issues. Because 5ARIs are lipophilic, they can cross the blood-brain barrier, potentially disrupting neurosteroid synthesis, altering neurochemistry, and impairing neurogenesis. These effects underscore the urgent need for further research into the long-term impact of 5ARIs and for innovative therapeutic solutions (Leliefeld et al., 2023). The complexity of these hormonal pathways underscores the importance of understanding the broader regulatory networks that influence cancer progression.
Furthermore, the link between obesity and prostate cancer has become increasingly evident, as obesity triggers pathways related to insulin resistance, chronic inflammation, and oxidative stress. These pathways, including the IGF-1 system, adipokine signaling, and the distribution of sex hormones, create a favorable environment for cancer initiation and progression (Birzniece et al., 2010; Birzniece et al., 2019). Insulin and IGF-1, both key players in metabolic regulation, share sequence similarities and can activate oncogenic signaling pathways such as MAPKs and PI3K-AKT, which are known to promote cancer cell proliferation and inhibit apoptosis. The dysregulation of these pathways in obesity enhances the autocrine and paracrine promotion of cancer, further complicating treatment strategies (O’Brien et al., 2005; Fogarty et al., 2008; Pollak, 2008; Avgerinos et al., 2019). Hormone-related cancers, particularly those affecting organs governed by intricate feedback mechanisms, are profoundly impacted when autocrine pathways, such as insulin-related signaling, become dysregulated. The activation of these feedback loops can amplify oncogenic signals, driving cancer progression and complicating treatment strategies (Park et al., 2022). Addressing this challenge requires targeted therapeutic approaches that restore and maintain homeostasis within these systems while mitigating the risks associated with hormonal and metabolic imbalances.
In addition to hormonal and metabolic factors, bone health is a critical consideration in prostate cancer management, especially given the high incidence of bone metastasis in advanced stages of the disease. Sustained ADT significantly reduces bone mineral density (BMD), leading to an increased risk of fractures and osteoporosis (Zhang et al., 2020). Studies show that BMD can decrease by between 4% and 13% annually during ADT, with the risk of fractures rising correspondingly (Miyazawa et al., 2018). To address these complications, it is essential to implement comprehensive bone health evaluations before initiating ADT. Recent guidelines have begun to prioritize bone health in prostate cancer management, recommending the use of bone-modifying agents (BMAs), such as bisphosphonates and denosumab, to mitigate bone loss and reduce fracture risk (Chakhtoura et al., 2021; Khan, 2023). However, patients with metastatic castration-resistant prostate cancer (mCRPC) have not seen an improvement in overall survival (OS) with bisphosphonates or denosumab. Nonetheless, having demonstrated the ability to postpone or avoid skeletal-related events (SREs) in patients with mCRPC, both BMAs have been approved in this context (Saad et al., 2004; Fizazi et al., 2011). About 90%–95% of patients with hormone-sensitive prostate cancer (mHSPC) have bone metastases (Fizazi et al., 2017; Parker et al., 2018). Unlike mCRPC, however, there is little data to support prescription of bone protective medicines in mHSPC (Cattrini et al., 2019). Notably, the lack of effective bone-protective agents in managing mHSPC underscores the urgent need to explore and develop novel therapies tailored to support bone health in prostate cancer patients. This complexity further highlights the necessity for multi-target therapeutic strategies capable of addressing the diverse mechanisms driving prostate cancer progression. By targeting metabolic dysregulation, hormonal feedback imbalances, and bone health simultaneously, multi-component approaches hold the potential to significantly enhance treatment efficacy, improve clinical outcomes, and ultimately elevate the quality of life for patients.
Next-generation anti-androgens like apalutamide, darolutamide, and enzalutamide have shown higher efficacy compared to first-generation agents, not only by competitively inhibiting the AR ligand-binding domain but also by preventing AR translocation to the nucleus and AR-mediated transcription (Crawford et al., 2018).
One of the most significant challenges in treating prostate cancer is the emergence of resistance to androgen receptor (AR)-targeted therapies. A major contributor to this resistance is the androgen receptor splice variant 7 (AR-V7), which lacks the ligand-binding domain necessary for traditional AR inhibitors to be effective (Antonarakis et al., 2016). AR-V7 remains constitutively active, driving cancer progression even in the absence of androgens. This splice variant has been detected in circulating tumor cells (CTCs) and is associated with resistance to androgen receptor axis-targeted agents (ARATs) such as enzalutamide and abiraterone (Antonarakis et al., 2016; Scher et al., 2016). Only a few of the more than 20 distinct AR variations that have been found have undergone in-depth research. The most extensively studied AR variations, outside AR-V7, are AR-V1, AR-V3, AR-V9, and ARv567es (Armstrong and Gao, 2019). For patients who express AR-V7, chemotherapy may prove more effective than AR-directed therapy, and ARATs combined with ADT remain standard treatment options for castration-resistant prostate cancer (CRPC) (Scher et al., 2012; Fizazi et al., 2014; Ryan et al., 2015; Hussain et al., 2018; Armstrong et al., 2019; Davis et al., 2019; Fizazi et al., 2019; Sternberg et al., 2020). The concurrent use of abiraterone acetate with low-dose prednisone is crucial to mitigate mineralocorticoid-related side effects, such as hypertension and fluid retention, further illustrating the complexity of managing advanced prostate cancer (Hatano and Nonomura, 2023).
Thus, therapeutic resistance remains a significant challenge in targeted cancer treatment due to tumor cell plasticity, which drives the emergence of resistance mechanisms such as target mutations, pathway reactivation, and interactions with the tumor microenvironment. Although targeted therapies hold great potential for personalized cancer treatment, the adaptability of tumor cells and their inherent heterogeneity often complicate treatment responses. However, a deeper understanding of these resistance mechanisms has led to the development of combination therapies, which are showing promise in improving therapeutic outcomes by overcoming resistance and enhancing treatment efficacy (Ramos and Bentires-Alj, 2015).
Innovative therapeutic strategies continue to emerge, including the exploration of tyrosine kinase inhibitors, vaccination therapies, immune checkpoint inhibitors (such as PD-1/PD-L1 and CTLA-4 inhibitors), PARP inhibitors, and PSMA-targeted treatments (Mitsogiannis et al., 2022). The heterogeneity of prostate cancer, particularly the plasticity induced by ADT, highlights the need for early multi-modal therapy that targets diverse mechanisms to prevent resistance and improve patient outcomes (Fizazi et al., 2017; James et al., 2017; Gravis et al., 2018; Kyriakopoulos et al., 2018). Prostate cancer patient data and overall disease progression indicate the significance of focusing on the network that comprises the main drivers. Thus, it is becoming clear that a more logical approach based on a more holistic strategy is to target the larger cancer-causing networks rather than to depend on single-target therapy. This approach attempts to interfere with the several processes that lead to tumor growth and resistance, acknowledging the complex nature of prostate cancer.
2 Challenges for targeting multiple pathways in prostate cancer therapeutics2.1 The role of DNMT1 in prostate cancer progressionThe rapid demethylation of 5-methylcytosine (5 mC) during epigenetic reprogramming, particularly during cancer progression, cannot be entirely explained by passive methyl loss during replication. Active enzymatic processes also play a crucial role in this demethylation, contributing to significant alterations in gene expression that fuel tumorigenesis (Wu and Zhang, 2010). Intratumoral heterogeneity (ITH) is increasingly recognized as a product of aberrant CpG methylation, which disrupts alternative splicing mechanisms and contributes to cancer’s adaptive and aggressive behavior (Lin et al., 2023). The interplay between genetic mutations and epigenetic modifications, such as DNA methylation and histone modifications, is a fundamental driver of oncogenesis (Network et al., 2013; Bullinger et al., 2017; Eisfeld et al., 2020), impacting key oncogenes and tumor suppressor genes to foster tumor progression and metastasis (Aguilera et al., 2010).
This detailed discussion brings to light how DNA methyltransferases (DNMTs), particularly DNMT1, are integral to this process (Elenbaas et al., 2001; Taube et al., 2013). For instance, DNMT1’s role in the epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) phenotypes highlights its significant impact on tumor initiation and progression (Lee et al., 2016). The co-expression of DNMT1 and the Enhancer of zeste homolog 2 (EZH2), alongside their correlation with poor prognostic markers in prostate cancer, underscores the importance of DNMT1 in maintaining tumor-promoting epigenetic landscapes (Li et al., 2022). Additionally, mechanisms involving other key proteins, such as calcium/calmodulin-dependent protein kinase II inhibitor I (CAMK2N1), which appears to be downregulated via promoter hypermethylation, further emphasize the role of DNMT1 in cancer (Peng W et al., 2023). This has therapeutic implications, as targeting DNMT1-mediated methylation could reactivate tumor suppressor genes like FAM107A, which is silenced in PCa through CpG island hypermethylation. FAM107A acts as a molecular brake on the FAK/PI3K/AKT pathway, and its reactivation could inhibit tumor growth and metastasis, offering a potential therapeutic target (Ke et al., 2022). Moreover, miRNAs, such as miRNA-148a and miRNA-125b, regulate DNMT1 and p53, influencing gene silencing and TP53-related pathways.
The therapeutic manipulation of these miRNAs in PCa could modulate DNMT1 activity and reverse oncogenic methylation patterns (Melnik, 2017). For example, mahanine, a plant-derived alkaloid, restores tumor suppressor gene expression by inhibiting DNMT1 degradation, revealing the therapeutic potential of natural compounds in targeting epigenetic machinery (Agarwal et al., 2013). Thus, the role of DNMT1 in the modulation of enhancer RNA (eRNA) linked to the androgen receptor (AR) suggests that targeting DNMT1 in these non-coding RNA interactions could provide new avenues for diagnostics and therapy. This complexity highlights the profound influence of epigenetic regulation in cancer biology, with DNMT1 as a central figure in the maintenance of oncogenic states (Pan et al., 2021). Nucleoside analogs cause DNA double-strand breaks and cell death, in addition to depleting DNMTs and lowering DNA methylation levels [12]. By reactivating endogenous retroviral elements, they also promote immunological responses via the viral defense pathway [21]. In prostate cancer, intratumoral heterogeneity (ITH) plays a critical role in driving local recurrence following radiation therapy. Emerging research highlights that the ecological interactions among distinct tumor cell subpopulations may significantly contribute to treatment resistance. This study aims to evaluate the impact of these intercellular dynamics on prostate cancer progression and their influence on the therapeutic response to radiation, providing new insights into the complexity of treatment resistance mechanisms (Paczkowski et al., 2021). Prostate cancer may exhibit reduced aggressiveness upon termination of DNMT1 expression, as this leads to a corresponding decrease in Enhancer of Zeste Homologue (EZH2) expression. DNMT1 promotes prostate cancer progression and metastasis by enhancing TRAF6 transcription and facilitating TNF receptor-associated factor 6 (TRAF6)-mediated ubiquitination of EZH2, underscoring its pivotal role in tumorigenesis and potential as a therapeutic target (Li et al., 2022). The development of epithelial-to-mesenchymal transition (EMT) and cancer stem cell (CSC) phenotypes within tumors is closely tied to the epigenetic regulation of genetic programs by DNA methyltransferases (DNMTs). In prostate cancer, EMT-driven bone metastasis is further facilitated by cancer-associated fibroblasts (CAFs), which enhance stromal CXC motif chemokine 12 (CXCL12) levels, creating a microenvironment that supports metastatic progression (Lee et al., 2016). The tumor suppressor gene calcium/calmodulin-dependent protein kinase II inhibitor I (CAMK2N1) is significantly downregulated in prostate cancer. This downregulation is driven by DNMT1-mediated DNA methylation, which not only suppresses CAMK2N1 expression but also triggers activation of the AKT and ERK signaling pathways. This activation establishes a feedback loop that promotes further DNMT1 production, amplifying oncogenic signaling and tumor progression (Peng Y et al., 2023). The FAM107A gene, located on the short arm of chromosome 3, is frequently downregulated in prostate cancer and is associated with poor prognosis. This downregulation is primarily driven by hypermethylation of CpG islands in its promoter region. Notably, overexpression of FAM107A has been shown to inhibit tumor cell motility, invasion, and proliferation while promoting apoptosis, primarily through modulation of the focal adhesion kinase (FAK)/PI3K/AKT signaling pathway (Ke et al., 2022). Milk-derived miRNAs, particularly miRNA-125b and miRNA-148a, influence the p53-DNMT1 regulatory axis, which governs key genes like BIRC5 (Baculoviral IAP Repeat Containing 5) (surviving) involved in cell survival and tumor progression. miRNA-125b targets TP53, altering p53-dependent gene networks, while miRNA-148a directly downregulates DNMT1, affecting chromatin regulation via Histone Deacetylase 1 (HDAC1). This milk-mediated miRNA-p53-DNMT1 pathway may explain the epidemiological link between milk consumption, acne vulgaris, and prostate cancer (Melnik, 2017). DNMT1 knockdown reduces repressive histone marks, particularly H3K9me3 and H3K27me3, on the promoters of Zinc Finger E-Box Binding Homeobox 2 (ZEB2) and Kruppel-like transcription factor 4 (KLF4) genes crucial for maintaining the EMT and CSC phenotype. This epigenetic alteration facilitates the transcriptional activation of these genes, driving aggressive tumor behavior and underscoring DNMT1’s pivotal role in modulating epigenetic landscapes within PCa cells (Lee et al., 2016). The multifaceted impact of DNMT1 across various signaling pathways and cellular mechanisms underlines its potential as a critical target in future prostate cancer treatments, particularly in strategies aimed at overcoming metastasis and resistance to conventional therapies. Table 1 summarizes the key points related to DNMT1’s role in prostate cancer.
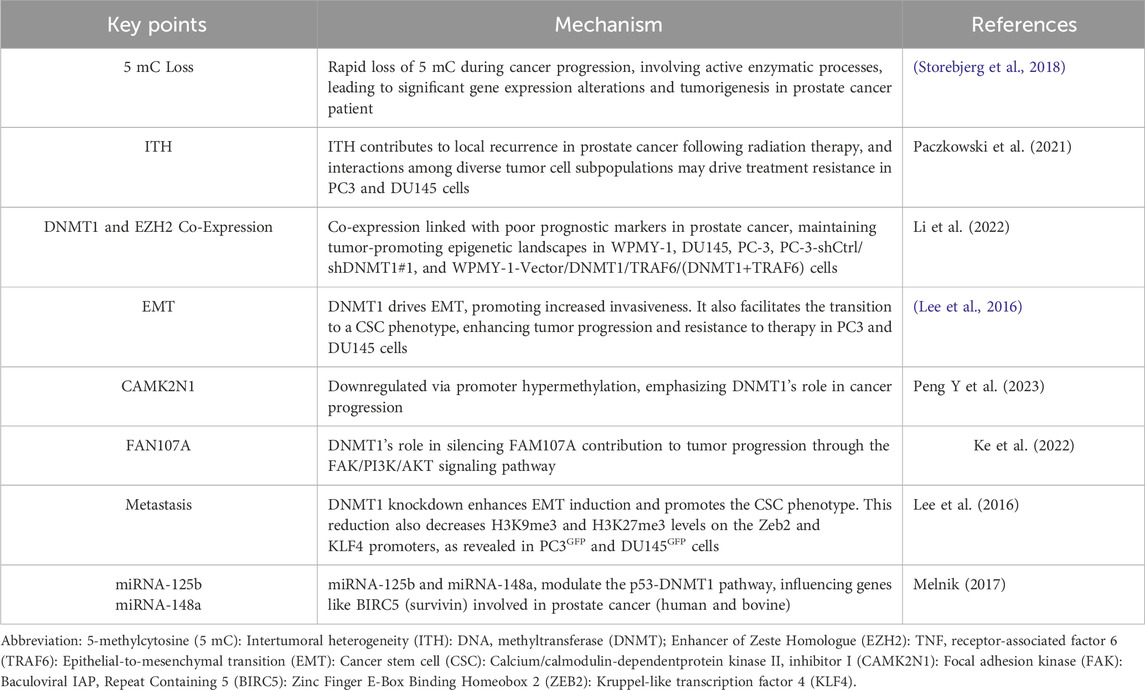
Table 1. The complex role of DNMT-1 in Prostate cancer.
2.2 The role of dicer in prostate cancer progressionDicer, an essential enzyme in the biogenesis of microRNAs (miRNAs), plays a crucial role in prostate cancer progression by regulating various cellular processes, including cell division, apoptosis, and tumor invasion. In prostate cancer, Dicer expression is notably elevated in cancerous tissues compared to benign counterparts, particularly in early-stage disease, with higher levels correlating to more aggressive phenotypes. Elevated Dicer and Ago2 expression in prostate cancer tissues compared to adjacent benign tissues have been linked to lower Gleason scores, suggesting a role in moderating tumor aggression. When Dicer or Ago2 expression is silenced in vitro, prostate cancer cell lines such as LNCaP, PC-3, and DU145 exhibit significant reductions in cell proliferation and increased cell death, indicating Dicer’s role in promoting tumor cell survival. Moreover, Dicer inhibition leads to cell cycle arrest in the G2/M phase in androgen-dependent LNCaP cells and in the S phase in androgen-independent PC-3 and DU145 cells, demonstrating its influence on cell cycle regulation across different prostate cancer subtypes (Bian et al., 2014).
Dicer dysfunction is observed across various cancer types, including prostate cancer, where altered miRNA processing contributes to disease progression. Dicer’s role in miRNA maturation enables miRNAs like miR-200a and miR-31, which are downregulated in prostate cancer tissues, to serve as potential diagnostic and prognostic markers. Interestingly, metastatic prostate cancer exhibits elevated expression levels of miR-200a, miR-370, and miR-31 compared to localized prostate cancer, suggesting Dicer’s differential regulation in advanced disease stages (Bian et al., 2015). Transforming growth factor-β1 (TGF-β1) plays a central role in regulating EMT through its influence on nc886, a non-coding RNA transcribed by RNA polymerase III (Pol III). nc886 affects EMT indirectly by modulating the processing of microRNAs via Dicer, an essential enzyme in RNA silencing. Additionally, TGF-β1 regulates MYC-associated zinc finger protein (MAZ), a transcription factor that suppresses TGFBI, a gene involved in cell adhesion and migration. This regulatory framework reveals a novel EMT unit comprising nc886 and its neighboring genes, driven by TGF-β1-mediated differential transcription of Pol II and Pol III genes. Understanding this network provides new insights into EMT regulation in PCa and highlights potential therapeutic targets for mitigating metastasis (Yang et al., 2022).
Disruption of Dicer function has been shown to increase apoptosis and senescence in prostate cancer cell models, driven by upregulation of tumor suppressors such as P16/INK4a and P27/Kip1. This suggests that Dicer acts as a survival factor in prostate cancer cells, contributing to the maintenance of tumor growth and resistance to apoptosis in PrEC cells, PNT1a and PNT2, LNCaP, PC-3, DU145, and CWR22Rv1 cells (Zhang et al., 2014). Immunohistochemical studies on prostate cancer tissues have demonstrated Dicer overexpression in prostatic intraepithelial neoplasia (PIN) and in over 80% of prostate adenocarcinomas, indicating its potential role as a biomarker for early detection and progression monitoring (Chiosea et al., 2006). Furthermore, hypoxic conditions, often present in the tumor microenvironment, exacerbate Dicer dysfunction, leading to the downregulation of critical miRNAs like miR-124 and miR-144, which are associated with autophagy and treatment resistance. Overexpression of these miRNAs in hypoxic conditions has been shown to enhance radiosensitivity by downregulating PIM1, a key factor in prostate cancer progression (Gu et al., 2016).
In addition to its role in miRNA biogenesis, Dicer is involved in androgen receptor (AR) reprogramming, particularly in the transition to CRPC. Overexpression of MIR222HGs promotes androgen-independent growth in HSPC LNCaP cells by suppressing androgen receptor activity and reducing the expression of key AR-regulated genes (KLK3, TMPRSS2, FKBP5), driving the transition toward a CRPC phenotype. This suggests that targeting Dicer and its associated miRNAs could provide novel therapeutic avenues for combating CRPC (Sun et al., 2018).
Here, Dicer is a pivotal regulator of prostate cancer progression, influencing key processes such as cell proliferation, apoptosis, miRNA biogenesis, and androgen receptor signaling. Its role in regulating miRNAs and involvement in epigenetic modifications highlights its potential as a therapeutic target and biomarker in prostate cancer. Further investigation into Dicer’s mechanisms could uncover new strategies for targeted therapies, particularly in advanced and treatment-resistant forms of prostate cancer. Table 2 concisely summarizes the intricate role of Dicer in prostate cancer, highlighting its potential therapeutic and diagnostic applications.
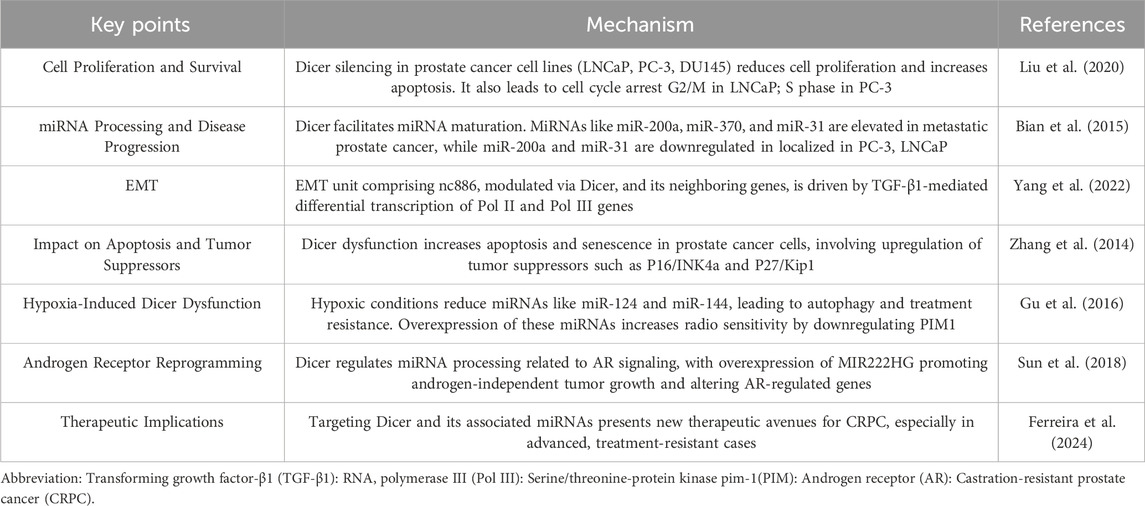
Table 2. The intricate role of Dicer in Prostate cancer.
2.3 PD-1/PD-L1 pathway in prostate cancerThe PD-1/PD-L1 pathway plays a critical role in immune evasion mechanisms employed by prostate cancer cells, making it a pivotal target in the development of immunotherapeutic strategies (Gerger et al., 2011). PD-L1, a transmembrane protein encoded by the CD274 gene, interacts with its receptor PD-1 on T cells, leading to the inhibition of T-cell activation and induction of T-cell anergy. This immune suppression enables tumor cells to evade immune detection and destruction. While targeting the PD-1/PD-L1 checkpoint has shown promise in a variety of cancers, including renal cell carcinoma, melanoma, and non-small cell lung cancer, prostate cancer, presents unique challenges due to its immunologically “cold” tumor microenvironment (Cha et al., 2019).
Collagen triple helix repeat containing 1 (CTHRC1) is associated with tumor progression and reduced disease-free survival in prostate cancer. High CTHRC1 expression correlates with increased levels of immune checkpoints PD-1 and PD-L1, enhanced infiltration of immune cells such as B cells, CD4⁺ T cells, macrophages, neutrophils, and dendritic cells, and is associated with genes like MMP9, MUC1, and SLC2B1 that drive PC progression. These findings suggest that CTHRC1 upregulation adversely affects PC prognosis and immune function, suggesting that targeting CTHRC1 could modulate the tumor microenvironment and improve therapeutic outcomes (Zhou et al., 2019).
Additionally, inhibition of heterogeneous nuclear protein L (HnRNP L) in castration-resistant prostate cancer (CRPC) reduces PD-L1 expression and destabilizes YY1 mRNA, thereby enhancing T-cell-mediated ferroptosis and improving antitumor immunity. This effect involves key molecules such as SLC7A11, Glutathione peroxidase 4 (GPX4), Signal transducer and activator of transcription 1(STAT1), Interferon gamma (IFN-γ), and Interleukin-1 (IL-2) (Zhou et al., 2022). Furthermore, PD-L1 protein levels in prostate cancer are regulated by proteasome-mediated degradation via Cyclin D-CDK4 and the Cullin 3SPOP E3 ligase pathway. Blocking CDK4/6 with inhibitors increases PD-L1 levels, enhancing immune suppression, which suggest that combining CDK4/6 inhibitors with PD-1/PD-L1 immunotherapies could disrupt tumor immune evasion and improve patient outcomes (Zhang et al., 2018).
Prostate cancer’s low presence of tumor-infiltrating lymphocytes (TILs) contributes to immunotherapies targeting immune checkpoints, such as anti-PD-1 or anti-PD-L1 antibodies (Sharma and Allison, 2015; Gao et al., 2017; Subudhi et al., 2020). EP4 (PTGER4), expressed in epithelial and immune cells, modulates the prostate cancer immune microenvironment. YY001, a novel EP4 antagonist, inhibits the differentiation and immunosuppressive function of myeloid-derived suppressor cells (MDSCs) while enhancing T-cell proliferation and anticancer activity. This agent reverses MDSC and T-cell infiltration by altering tumor chemokine profiles, leading to increased CD8+ T-cell activation and reduced immunosuppressive functions (Peng et al., 2022).
Clinical studies have shown that docetaxel-based chemohormonal therapy increases tumor-infiltrating T cells by activating the cGAS/STING pathway and inducing IFN signaling. In xenograft mouse models, this therapy enhances T-cell infiltration and upregulates PD1/PD-L1 expression, sensitizing tumors to anti-PD1 blockade. A retrospective analysis of metastatic castration-resistant prostate cancer (mCRPC) patients demonstrated improved PSA progression-free survival with combined docetaxel and anti-PD1 therapy compared to anti-PD1 alone (Ma et al., 2022).
In metastatic castration-resistant prostate cancer (mCRPC), single-agent PD-1/PD-L1 inhibitors have shown limited efficacy, underscoring the need to “heat up” these tumors by enhancing immune cell infiltration (Huang and He, 2020). Strategies to convert “cold” tumors into “hot” tumors (those with increased T-cell infiltration and immune activity) are being actively investigated. One promising approach is the combination of PD-1/PD-L1 inhibition with therapies that target other immune-modulatory pathways, such as CXCR4, poly (ADP-ribose) polymerase (PARP), or transforming growth factor (TGF)-β inhibitors. Combining these therapies with immune checkpoint inhibitors has shown potential in enhancing antitumor responses and overcoming immune resistance (Majidpoor and Mortezaee, 2021).
Additionally, integrating PD-1/PD-L1 inhibitors with chemotherapy, radiation therapy, or other targeted therapies offer new opportunities for prostate cancer treatment. For instance, docetaxel (DTX) induce ATM-NF-κB signaling, upregulating PD-L1 expression and contributing to immune suppression. Combining DTX with PD-1/PD-L1 inhibitors may counteract this effect and restore immune system activity for more effective tumor control (Wang et al., 2021).
Using the Myc-CaP:PSMA (+) murine prostate cancer model and second-generation anti-hPSMA CAR T cells with a Click Beetle Red luciferase reporter, researchers evaluated CAR T cell trafficking and antitumor efficacy both alone and in combination with anti-PD-1 antibodies. They found that combining CAR T cell therapy with PD-1 blockade reversed the exclusion of CD3+ T cells from tumor centers and enhanced tumor treatment response, although the effect was short-lived. Additionally, an inverse pattern of CAR T cell bioluminescence was observed in treated tumors, linked to decreased mitochondrial function following T cell activation, highlighting metabolic challenges in solid tumor therapies (Serganova et al., 2017).
Clinical trials continue to explore the benefits of combining PD-1/PD-L1 inhibition with other therapeutic agents, such as tyrosine kinase inhibitors, PARP inhibitors, or radiotherapy, to enhance immune responses and improve clinical outcomes. Understanding the intricate regulation of PD-L1 expression and the immune microenvironment in prostate cancer is crucial to developing more effective and personalized treatment strategies (Rekoske et al., 2016). Although the “cold” immune environment of prostate cancer limits the effectiveness of PD-1/PD-L1 inhibitors as monotherapy, combination therapies hold significant potential. Ongoing research aims to uncover the mechanisms driving immune evasion in prostate cancer and develop novel therapeutic strategies that enhance immune cell infiltration and activity, ultimately improving patient outcomes in this challenging disease (Sharma et al., 2020).
To consolidate advances in immunotherapy, it is crucial to identify candidate agents that not only enhance our immune system but also maintain an overall balance. Such agents could transform the immune environment, potentially converting immunologically “cold” tumors into “hot” ones, thereby increasing the effectiveness of immune responses against cancer. Table 3 highlights the major points discussed in the text, providing an overview of the challenges, mechanisms, and potential strategies related to PD-1/PD-L1 immunotherapy in prostate cancer.
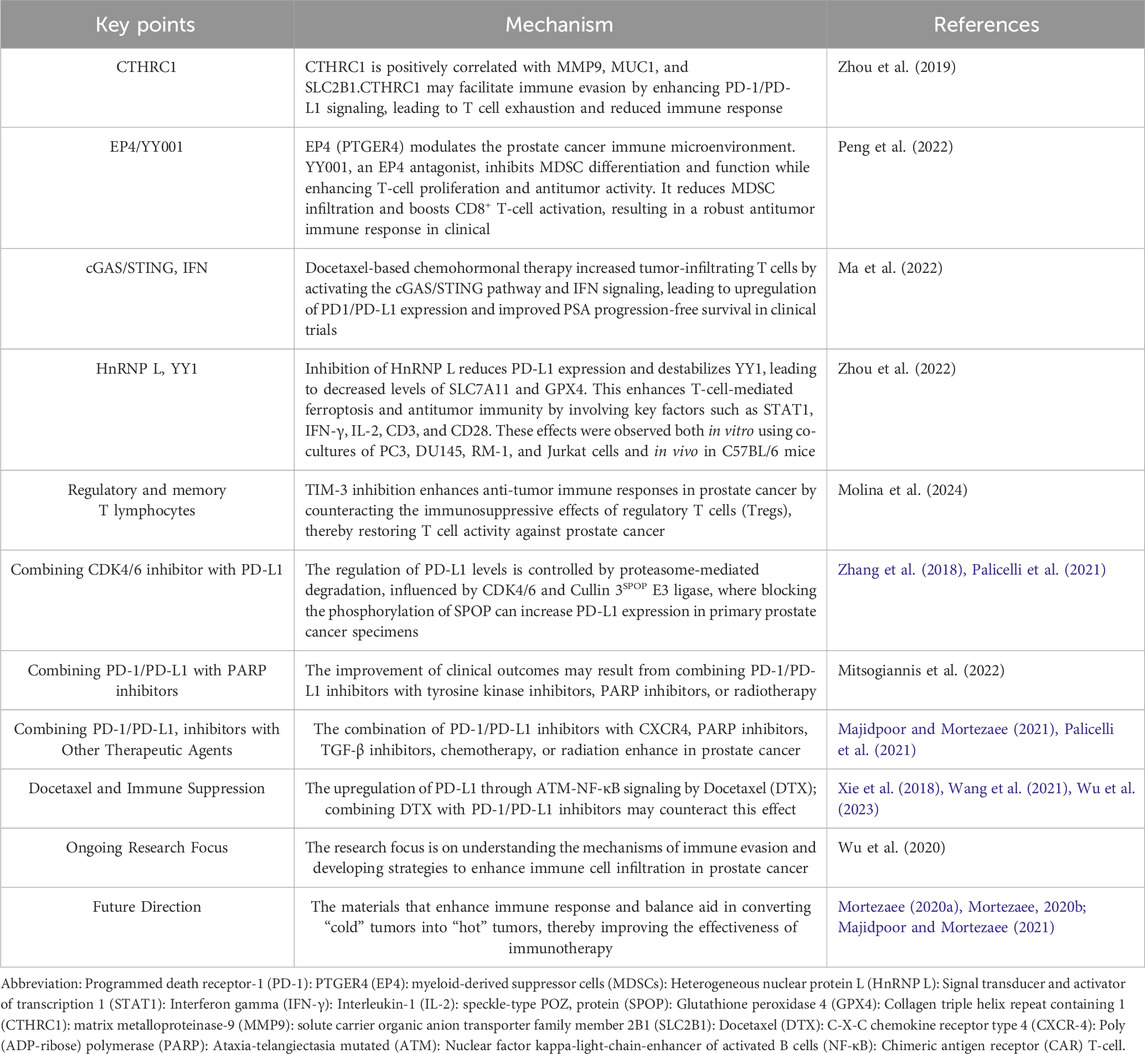
Table 3. The potential stages related to PD-1/PD-L1 immunotherapy in Prostate cancer.
3 A promising herbal medicine3.1 BK002 bioinformatics and network pharmacology analysis targeting DNMT1, dicer, PD-L1, and PD-1 in prostate cancerFigure 2A shows the KEGG pathway alignment for BK002 (a combination of AJN and MFR), specifically targeting DNMT1, Dicer, PD-L1, and PD-1. KEGG (Kyoto Encyclopedia of Genes and Genomes) was used to map gene interactions and pathways, highlighting which genes involved in these pathways are influenced by the bioactive compounds in AJN and MFR. The gene sets corresponding to DNMT1, Dicer, PD-L1, and PD-1 were obtained from publicly available gene databases (e.g., NCBI, KEGG). Figure 2B illustrates the interconnected network of KEGG pathways influenced by the compounds in AJN and MFR, showing how they interact with multiple prostate cancer-related pathways. Genes involved in the targeted pathways (DNMT1, Dicer, PD-L1, PD-1) were mapped with their interaction partners. The network shows direct and indirect connections between these pathways and other cancer-related genes regulated by AJN and MFR compounds. Figure 2C displays a comparative analysis of how AJN and MFR compounds affect multiple pathways on average, demonstrating the overall balance in their pharmacological action on prostate cancer pathways. KEGG pathways affected by AJN and MFR compounds were averaged based on the number of overlapping genes. This approach helps in identifying whether the two compounds target pathways with similar intensities or whether one has a stronger influence. Figure 2D highlights the average interaction network based on the aligned pathways of AJN and MFR. It aggregates the interaction data, presenting a more generalized view of how the two herbal medicines interact with the prostate cancer gene pathways. Using network pharmacology tools, the average alignment of the networks for AJN and MFR was computed. Nodes represent genes or proteins, while edges represent interactions or relationships between them.
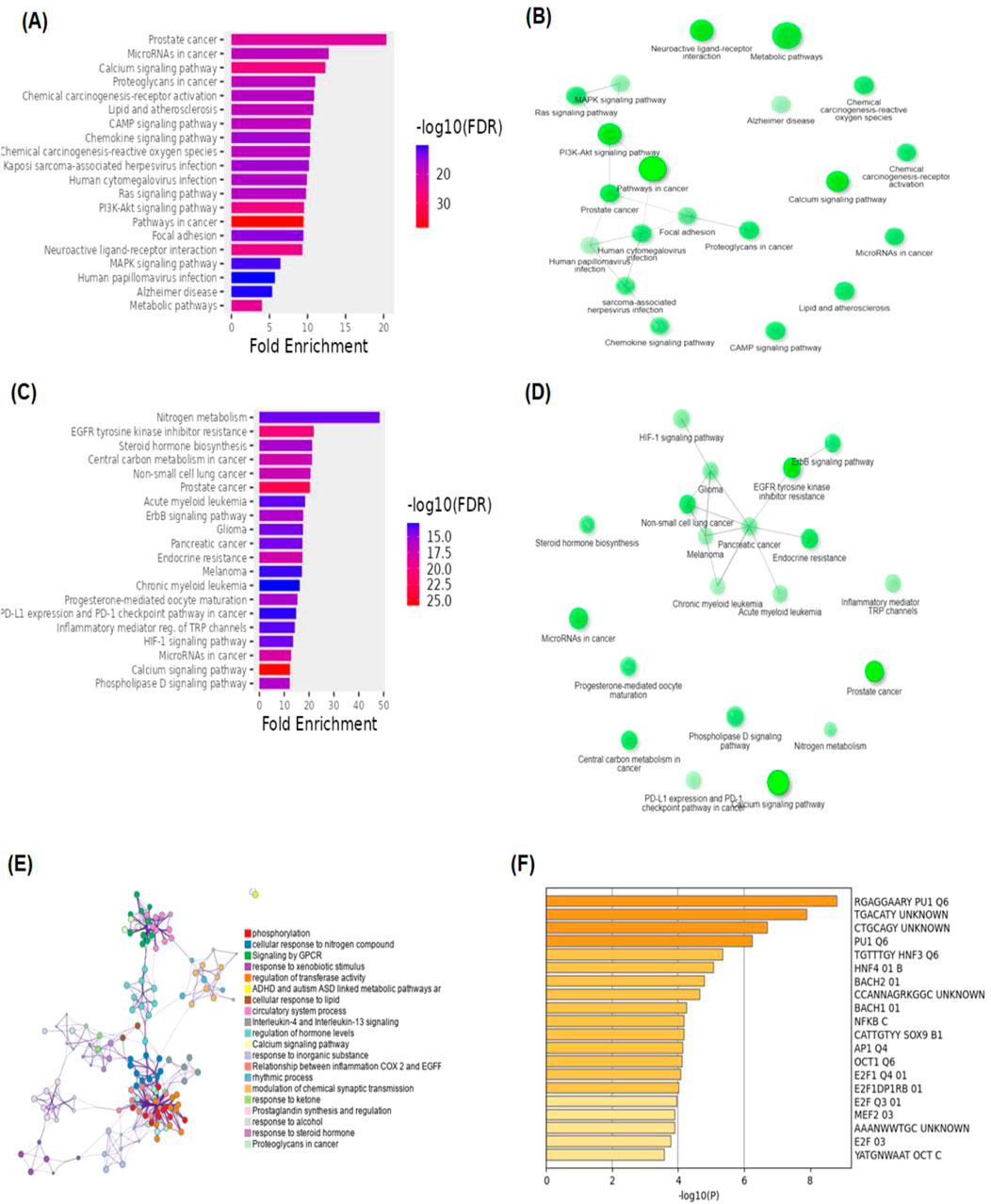
Figure 2. BK002 Network Pharmacology Analysis in Prostate cancer. (A) Gene alignment KEGG pathway of AJN and MFR, (B) Gene alignment KEGG pathway network, (C) Average equality alignment KEGG pathway of AJN and MFR, (D) average alignment KEGG pathway network, (E) overall network of AJN and MFR and (F) target transcription factors of AJN and MFR.
Figure 2E presents the comprehensive interaction network of AJN and MFR compounds across all the targeted pathways in prostate cancer, including DNMT1, Dicer, PD-L1, and PD-1. It reflects the complete bioinformatics data from pathway analysis. Network pharmacology platforms (such as STITCH, STRING, or Cytoscape) were used to generate a global interaction map. This figure provides an integrative view, demonstrating the full therapeutic potential of these herbal extracts. Figure 2F focuses on the specific transcription factors regulated by AJN and MFR compounds, which are crucial in modulating the expression of DNMT1, Dicer, PD-L1, and PD-1 in prostate cancer. Transcription factors known to regulate the expression of DNMT1, Dicer, PD-L1, and PD-1 were identified using databases like TRANSFAC or JASPAR. AJN and MFR compounds were then analyzed to determine their influence on these transcription factors through docking simulations or network modeling.
3.2 Ethnopharmacological background of herbal medicine in prostate cancerTraditional cancer treatments like surgical resection, radiotherapy, and chemotherapy have been fundamental in oncology. However, the advent of immunotherapy and targeted therapies has significantly enhanced cure rates. Alongside these advancements, increasing clinical and laboratory evidence supports the efficacy of herbal medicines in cancer treatment (McCubrey et al., 2017). Phytochemicals from herbal sources exhibit notable anticancer properties, complementing conventional therapies (Xiang et al., 2019). Traditional herbal medicine, with its millennia-long history, is experiencing renewed scientific interest, with modern methods validating its efficacy, safety, and mechanisms of action (Chen L et al., 2024). For instance, during the COVID-19 pandemic, around 90% of patients in China used traditional herbal medicine, achieving an 80% effectiveness rate with minimal side effects (An et al., 2021). In Asia, herbal medicine is widely used alongside conventional cancer treatments (Li et al., 2013; Xu et al., 2020). Benefits include enhanced immunity, symptom relief, and improved quality of life. Herbal extracts and formulations target multiple pathways to combat drug resistance, induce tumor apoptosis, and inhibit tumor growth (Jin et al., 2021; Lee et al., 2021; Lu et al., 2021; Wang et al., 2024).
Notably, plant-derived phytochemicals and extracts exhibit different mechanisms of effectiveness against prostate cancer (Huang et al., 2019; Livingstone et al., 2019; Wang et al., 2019; Bai et al., 2021; Ghosh et al., 2021; Singla et al., 2021; Ruksiriwanich et al., 2022; Kong et al., 2023; Peng W et al., 2023; Chen M et al., 2024; Huang et al., 2024; Ji et al., 2024). Research in systems biology will undoubtedly help modernize herbal medicine and establish the multicomponent, multitargeting approach as a new paradigm in medicine. Once identified, metabolic engineering and/or chemical synthesis can be used to produce the active constituents of a herbal medicine more efficiently (Kim et al., 2015). These treatments leverage a variety of bioactive compounds like saponins, triterpenes, phenolics, and flavonoids, known for their antimicrobial, anti-inflammatory, immunomodulatory, antioxidant, and anticancer effects (Batiha et al., 2023). The growing acceptance and integration of herbal medicines in cancer care reflect their potential to enrich and enhance therapeutic outcomes, offering a holistic approach to oncology.
3.3 Achyranthes japonica (Miq.) Nakai (AJN)AJN, a perennial herb in the Amaranthaceae family, is prevalent in East Asia, including Korea, China and Japan (Jung et al., 2007). Traditionally used to treat edema, arthritis, mastitis, and delayed menstruation (Marcone et al., 2003). Recent studies have revealed that the phenol content of AJN reached its peak at a sowing amount of 0.5 g, while the flavonoid content is maximized at both 0.5 and 1.0 g, underscoring the ideal conditions for bioactive compound production (Kim et al., 2024). Notably, AJN is rich in phytochemicals such as saponins, inokosterone, ecdysterone, and oleanolic acid bisdemoside (Hahn and Lee, 1991; Ida et al., 1994). These compounds contribute to its diverse biological and pharmaceutical activities, including anti-inflammatory, antioxidant, antimicrobial, osteoprotective (Jung et al., 2007; Bang et al., 2012; Park et al., 2013), anti-diabetic, and anticancer (Shim et al., 2016). Dietary supplementation with AJN extract in animal models has improved growth performance, nutrient utilization, intestinal microbiota balance, and reduced excreta ammonia levels (Sun H. Y et al., 2020). Recent studies highlight the potential of AJN in various therapeutic applications. AJN, particularly in its fermented form, has shown significant effects in animal models of osteoarthritis, reducing inflammation and catabolic factors while preserving joint architecture (Kim D et al., 2020).
AJN extract demonstrated anti-allergic effects by suppressing histamine release and intracellular calcium [Ca2+]i elevation in FcɛRI-mediated KU812F cells in a dose-dependent manner. Flow cytometry revealed reduced FcɛRI surface expression and decreased binding of IgE to FcɛRI. Additionally, AJN extract downregulated FcɛRI α chain mRNA levels, suggesting its mechanism involves FcɛRI expression inhibition, calcium influx suppression, and histamine release reduction (Shim et al., 2016).
In a monosodium iodoacetate (MIA)-induced osteoarthritis animal model, dietary supplementation with fermented AJN (FAJN) reduced serum prostaglandin E2 (PGE2), proinflammatory cytokines, and cartilage catabolic factors such as MMP-3 and MMP-7. These findings suggest that FAJN may have therapeutic potential for managing osteoarthritis (Kim H. Y et al., 2020).
AJN root (AJNR) demonstrated specific effects on IL-6-mediated catabolic and anabolic alterations, reducing catabolic factors and recovering anabolic factors in vitro. In a destabilization of the medial meniscus (DMM) model, AJNR decreased cartilage erosion, subchondral plate thickness, and osteophyte size and maturity. In a CIA model, AJNR effectively inhibited cartilage degeneration, synovium inflammation, and pannus formation in the ankle and knee. Immunohistochemical analysis revealed its primary action involved suppressing IL-6-mediated matrix metalloproteinase-3 and -13 in arthritis models (Zhao et al., 2021). AJN has shown promising results in reducing NO and PGE2 production in LPS-induced cells and in decreasing MMP-3 release in TNF-α-treated cells. Fermented AJN exhibits enhanced anti-inflammatory activity and greater concentrations of active components (Lee et al., 2012). Notably, combined extracts of AJN with other herbs have shown significant anti-inflammatory effects, reducing the production of pro-inflammatory cytokines and showing potential in managing osteoarthritis and other inflammatory conditions. AJN’s inhibitory effects on NF-κB activation and ERK, JNK, and p38 phosphorylation further underscore its therapeutic potential (Bang et al., 2012).
In prostate cancer models, BK002 (AJN combined with MFR) increased DNA damage and activated p-γH2A.X, promoting ubiquitination of pro-PARP, caspase9, and caspase3, leading to apoptosis in PC3 and DU145 cells. Confocal imaging showed enhanced DNA-binding activity, while BK002 also induced CHOP activation and suppressed PI3K/A
Comments (0)