Dyspnoea, or breathlessness, is the subjective experience of breathing discomfort, characterised by distinct sensations of varying intensity as described by the American Thoracic Society.[1] As the severity of cardiac and pulmonary diseases progresses, the frequency of dyspnoea increases,[2,3] yet this distressing symptom often remains inadequately managed.[4]
Dyspnoea not only limits physical activity, exacerbating breathlessness through physical deconditioning, but it also establishes a cycle that severely affects patients’ quality of life. Its presence is linked to heightened anxiety and depression, frequent hospitalisations and increased mortality.[5] This burdensome symptom is especially prevalent in palliative care, with rates of 50–70% among patients with advanced cancer and reaching as high as 90% in those with lung cancer.[6]
To address the multifaceted nature of dyspnoea, the Multidimensional Dyspnoea Profile (MDP) was introduced by Banzett et al. in 2015. This tool is structured to assess both the sensory and emotional dimensions of breathlessness, providing a comprehensive evaluation of the symptom’s impact.[7] Since its development, the MDP has been translated into several languages, such as Swedish,[8] French, Japanese, Spanish and, more recently, Hindi.[9]
However, no validated Telugu version of the MDP currently exists, despite Telugu being spoken by approximately 75 million people in Andhra Pradesh and Telangana. Given the high illiteracy rates in this population and the critical need for accessible tools within palliative care, a Telugu adaptation of the MDP is both timely and essential.
AimThe aims of this study were to translate, culturally adapt and linguistically validate the MDP for effective use among Telugu-speaking populations in Indian palliative care settings, with consideration for high illiteracy rates.
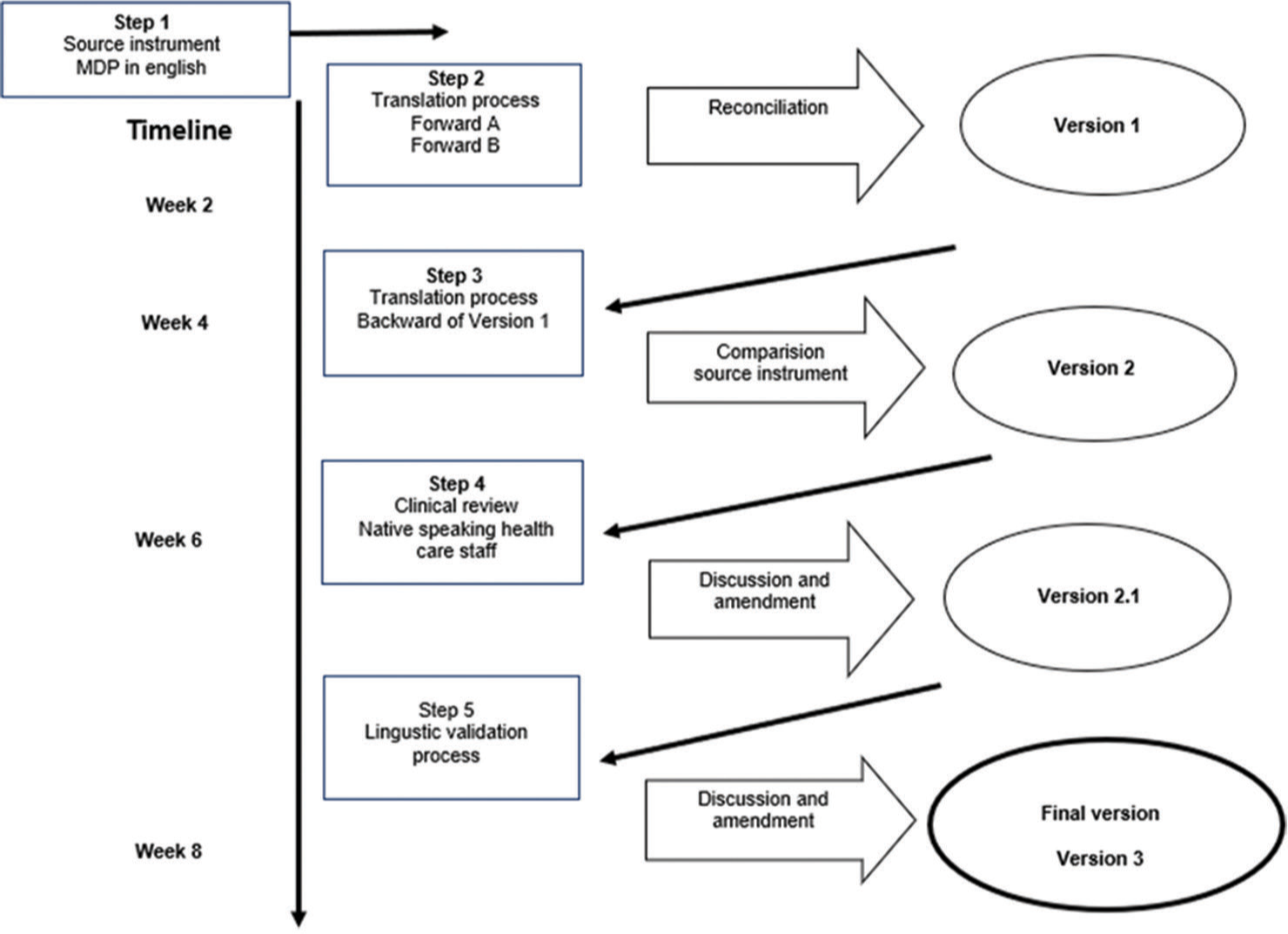
MATERIALS AND METHODS Translation and linguistic validation processThe MDP was systematically translated from American English to Telugu in partnership with the Language Services Unit at Société par Actions Simplifiée [Figure 1]. Mapi is known for its expertise in translating and validating patient-reported outcome (PRO) instruments.
Export to PPT
Ethical review and consentThe Ethics Committee at Mehdi Nawaz Jung Institute of Oncology and Regional Cancer Center (MNJIORCC) in Hyderabad, India (2022), approved this study. Patients and their families were verbally briefed by healthcare providers and written or fingerprint-signed consent was obtained from all participants, with witnesses and healthcare providers also signing the consent forms.
MethodologyFollowing the translation guidelines of Mapi Research Trust (accessible online at Clinical Outcome Assessments (COA) Translation and Linguistic Validation | Mapi Research Trust, mapi-trust.org), the MDP underwent a rigorous, multistage translation and validation process in accordance with international standards.[10,11] After approval for translation, two certified Telugu translators from Translated S.R.L., Rome, Italy, produced initial Telugu versions independently. A third translator then reconciled these versions to create Version 1, which was back-translated into English and reviewed by an Indian linguistic consultant. After necessary modifications, Version 2.0 was produced.
Review by cliniciansThe second version (Version 2.0) was evaluated by two native Telugu-speaking physicians (authors GP and SR) at MNJIORCC, who assessed its clarity and relevance for Telugu-speaking patients with dyspnoea, leading to a refined Version 2.1 for use in the study.
Participant criteriaPatients in palliative care, either at home or at Kumudini Devi Hospice, were selected based on specific criteria: Age 18 or older, native Telugu speakers, experiencing recent significant breathlessness and capable of answering questions. Patients with cognitive impairments or those who were non-native Telugu speakers were excluded from the study.
Linguistic validation processVersion 2.1 of the translated MDP was administered orally to patients by healthcare staff in a single session, with responses recorded by experienced palliative care nurses or physicians according to a standardised protocol. Cognitive interviews were conducted to assess clarity, comprehensibility and suitability of the MDP. Appendix 1 includes the validated questions for patients, and participants provided feedback on their understanding of each item, suggesting alternative wording for any unclear phrasing [Appendix 2].
Healthcare staff conducting patient interviews were later interviewed to discuss any issues or misunderstandings observed in patient responses. Appendix 3 contains the validated questions for staff, who commented on areas where patients may have experienced confusion and offered alternative phrasing suggestions [Appendix 2]. After careful review and adjustments, the finalised Telugu MDP (Version 3) was completed and is detailed in Appendix 4. The sample size is determined by reaching data saturation.
RESULTS Study outcomes Patient profileThe study involved 24 cancer patients diagnosed with various types, including head and neck (n = 5), breast (n = 5), gynaecological (n = 4), lung (n = 4), haematological (n = 3), gastrointestinal (n = 2) and prostate (n = 1) cancers. The group included nine males and 15 females, with an average age of 49.5 years. Dyspnoea severity, measured using the Shortness of Breath Questionnaire (SQ) with a scale from 0 to 10, showed varying intensities among participants. Fourteen participants received in-home care, and the rest were in hospice care. More demographic details are shown in Table 1.
Table 1: Demographics in patient group (n=24).
Variable Number (%) Literate Yes 14 (58) No 10 (42) Education No education 9 (37.5) 1–10 years 11 (45.9) 11+ years 4 (16.6) Employment Unemployed 13 (54.2) Daily labourer 3 (12.5) Employed 4 (16.6%) Retired 2 (8.3%) Household income (per/month, INR) 5000–10000 1 (4.2) >10000 1 (4.2) White card Yes 5 (20.9) No 4 (16.6) Translation review and revisionsFollowing clinician feedback, additional changes were made to the initial translation, with a focus on enhancing the clarity of terms in the MDP’s second domain.
Validation resultsThe validation process engaged 24 Telugu-speaking patients, with 14 in palliative home care (58%) and 10 at Kumudini Devi Hospice (42%). A majority (83%) completed the entire questionnaire, and all responded to validation questions. In addition, three doctors and one nurse evaluated the instrument’s applicability. Most patients (88%) reported that the MDP questions were easy to understand, although 3 (12%) encountered difficulties. Four patients (17%) gave inconsistent responses in the second SQ step, and 9 (38%) selected ‘does not apply’ for some SQ symptoms while rating them higher on the scale.
All healthcare staff (4/4) indicated that the MDP helped them better understand patients’ dyspnoea and found it useful for both home and hospice care. However, some items were challenging for patients with limited education, potentially causing response inconsistencies. Staff also noted that the MDP’s comprehensiveness could be time-intensive, particularly for critically ill patients.
Final modificationsBased on staff feedback, the ‘radio metaphor’ from the American English MDP was removed due to its complexity for patients with limited education [Appendix 5]. While initially included in Version 2.1, patient feedback revealed misunderstandings, leading to its exclusion in the final version. Further refinements included simplifying terms and using symbols (e.g. replacing ‘or’ with ‘/’). The term ‘depression’ was also modified for clarity, with a Telugu equivalent added alongside the English term in Telugu script. With these adjustments, the final Telugu MDP (Version 3) was approved by the Mapi Institute on 28 August 2023, and is available in their database for clinical and research use.
For details on specific revisions made, please refer to Appendix 2.
DISCUSSIONThis study presents a linguistically validated Telugu version of the MDP, designed to support a comprehensive assessment of breathlessness in a palliative care context serving a largely underserved population with high illiteracy rates.[12-14] The translation followed international guidelines for PROs as outlined by the Mapi Institute, recognised for its expertise in cross-cultural validation of such tools.
This research is among the initial efforts to adapt the MDP for a palliative care setting, where specific adaptations were necessary. During earlier work translating the MDP into Hindi, challenges were identified that helped inform the Telugu adaptation process.[9] A significant issue noted with both translations is the MDP’s length and complexity, which may be demanding for critically ill patients. In high-intensity care settings, using selected sections of the MDP, rather than the entire tool, may help reduce demands on patients and healthcare providers alike.
The linguistic validation involved Telugu-speaking patients from socioeconomically disadvantaged backgrounds, many with limited formal education, which influenced their ability to engage with certain rating scales and abstract concepts.[15,16] For example, the numeric rating scale (NRS) was challenging for some patients when evaluating symptom severity. To address this, local healthcare staff suggested using a familiar concept – the Indian Rupee – as a reference point, where 10 rupees indicated high symptom severity, and half a rupee indicated a moderate level. Further simplifying the language in the MDP could improve accessibility for patients with limited educational backgrounds.
CONCLUSIONThis study resulted in a linguistically validated Telugu MDP, offering a useful tool for assessing dyspnoea in Telugu-speaking patients in palliative care. The MDP provided helpful insights into factors contributing to breathlessness in this population. However, given the time-intensive nature of the tool, selectively using sections as needed may help reduce the burden. Simplifying the language further may also be essential to ensure that patients with limited education fully comprehend the MDP questions, maximising its utility in this context.
Comments (0)