PIWI-interacting RNAs, called piRNAs, are single-stranded small RNAs with a size of 24–31 nucleotides (Iwasaki et al., 2015). They are bound by a special class of proteins, namely, PIWI - an abbreviation of P-element Induced Wimpy testis in Drosophila. (Lin and Spradling, 1997; Sohn and Oh, 2023). The piwi gene was first identified to be involved in germline stem cell division in Drosophila gonads, in both ovaries and testis (Lin and Spradling, 1997). Subsequently, piRNAs were discovered in mouse testis in 2006 and were defined as small non-coding RNAs that specifically interact with PIWI proteins (Aravin et al., 2006; Girard et al., 2006). For distinction, the name Piwi refers to one of the specific proteins in Drosophila, while PIWI refers to the protein subfamily (Ross et al., 2014). Additionally, PIWIs belong to the Argonaute proteins family, which encompasses another branch containing Ago proteins that bind to a different class of small RNAs, specifically, microRNAs (miRNAs) and small interfering RNAs (siRNAs) (Wang X. et al., 2023) (Figure 1A). Like the fellow members of Argonaute protein family, PIWIs have a bilobal structure, with lobes at both the N- and C-terminus binding to the 5′ and 3′ ends of piRNAs, like a pocket, protecting the piRNA (Matsumoto et al., 2016; Dayeh et al., 2018). The PIWI proteins are well conserved among different classes of animal species (Kim et al., 2018; Huang and Wong, 2021) (Table 1). The PIWI proteins consist of four main domains, namely, PIWI-Argonaute-Zwille (PAZ), N-terminal (N), PIWI, and middle (MID). The PAZ domain recognizes and binds to the 2′-O-methylated 3′end of piRNA, while the MID domain binds to the 5′uridine end, and the PIWI domain acts as an RNase H endonuclease to cleave the target transcript captured by piRNA through complementary base pairing (Litwin et al., 2017) (Figure 1B).
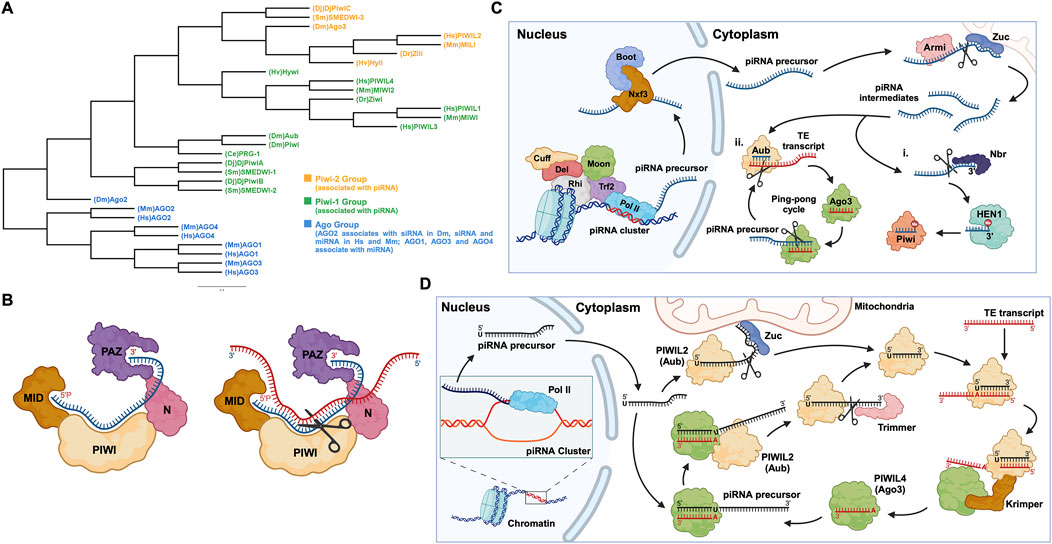
Figure 1. PIWI proteins and their functional domains in the piRNA-PIWI complex, and the overview of the piRNA pathway in Drosophila. (A) Phylogenetic tree of the Argonaute (AGO) protein family with each class of AGOs specific to the type of small non-coding RNAs. Hs: Homo sapiens, Mm: Mus musculus, Sm: Schmidtea mediterranea, Dr: Danio rerio, Hv: Hydra vulgaris, Dm: Drosophila melanogaster, Dj: Dugesia japonica, Ce: Caenorhabditis elegans. (B) (left) The MID domain of PIWI proteins binds to the 5′phosphate group of piRNA (shown in blue), and the PAZ domain binds to the 2′-O-methylated 3′end of piRNA (right). The target RNA is captured by piRNA through base-pair complementarity, and the PIWI domain, which possesses ribonuclease activity, cleaves the target RNA (shown in red). (C) The piRNA biogenesis in Drosophila: First, the piRNA cluster on the chromosome is transcribed by the RNA Pol II to produce piRNA precursors with the assistance of proteins, namely Rhino (Rhi), Cutoff (Cuff), Deadlock (Del), Trf2, and Moonshiner (Moon). The piRNA precursors are transported out of the nucleus by proteins such as Nxf3 and Bootlegger (Boot), and their secondary structure is removed by Armitage (Armi), and cut by Zucchini to form piRNA intermediates. Following, some piRNA intermediates, after being trimmed by Nibbler (Nbr) and methylated at the 3′end by Hen1, bind to Piwi (PIWIL1 homolog) to form a Piwi-piRNA complex (i). Other piRNA intermediates bind to Aub (PIWIL2 homolog) and then enter the ping-pong cycle dominated by Aub and Ago3 (PIWI4 homolog) (ii). (D) The ping-pong cycle, widely conserved in zebrafish, mice, humans, and other animals, silences target RNAs (such as TE transcripts) and amplifies piRNAs. The initial piRNA precursor, transcribed by RNA Pol II is exported from the nucleus to the cell cytoplasm, where it is processed into a mature form. After binding to the Aub protein, the mature piRNA cleaves the target TE transcript. The fragments of the cleaved transcript, bound by the Ago3 protein, act as a template and facilitate the processing of the piRNA precursor. The processed piRNA precursor, bound by the Aub protein and cleaved with the help of the protein Trimmer, enters the cycle to continue the silencing of complementary transcripts and the generation of piRNAs.
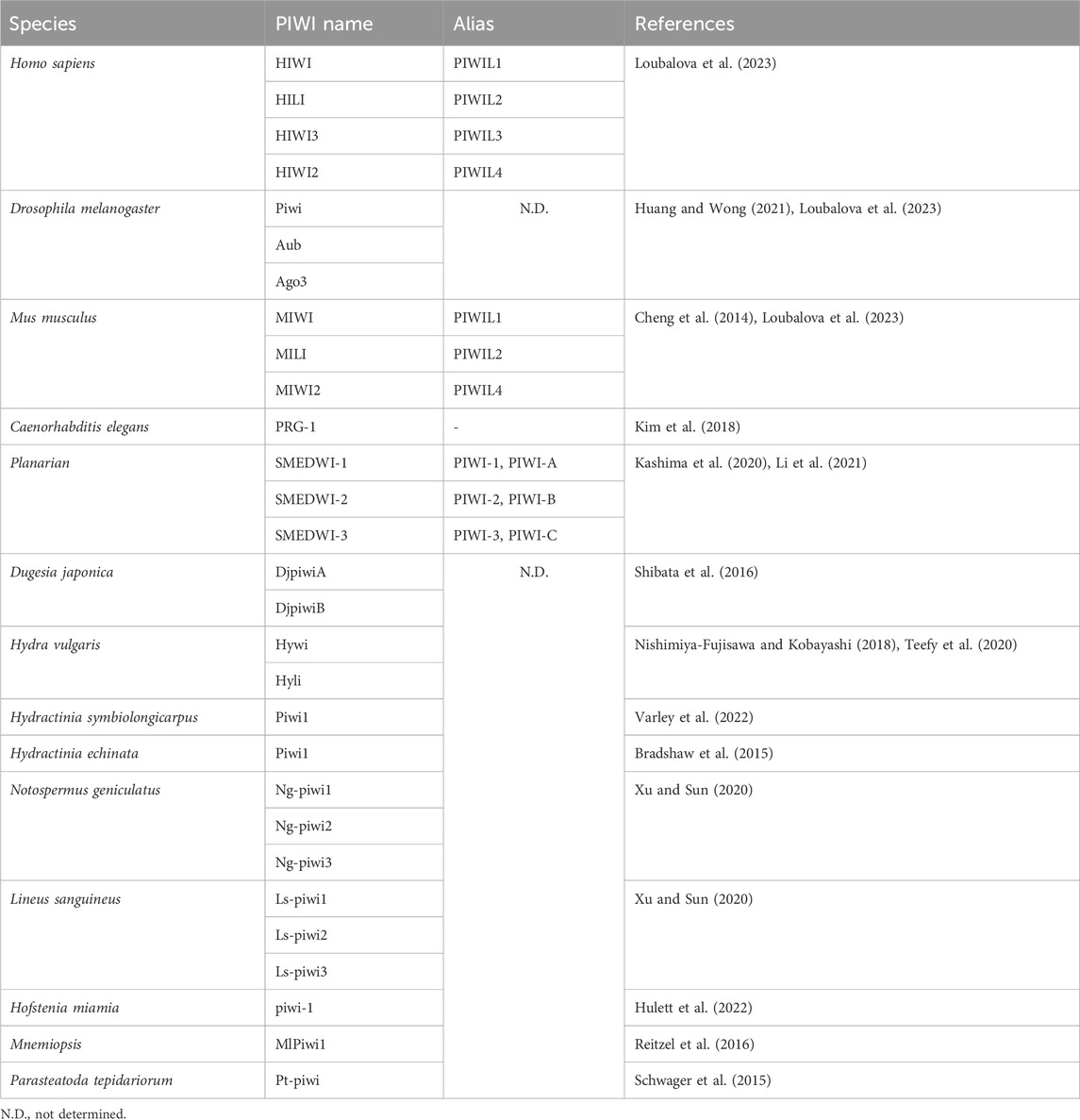
Table 1. Nomenclature of the PIWI proteins in different organisms.
1.2 piRNA biogenesis and the formation of piRNA-PIWI complexpiRNAs are generated from specific gene loci called piRNA clusters (Parhad and Theurkauf, 2019). In the cell nucleus, piRNA clusters are transcribed by RNA Polymerase II (RNA Pol II), into piRNA precursors, then these transcripts are exported to the cytoplasm through nuclear pores and are processed by enzymes such as Zucchini to form smaller piRNA fragments called piRNA intermediates (Huang and Wong, 2021; Ramat and Simonelig, 2021; Allikka Parambil et al., 2024). Most of these fragments are bound by the homologous protein PIWIL2, enter the ping-pong cycle (a process described in detail later on, in this review), or are methylated at the 3′end by the methyltransferase Hen1, forming a mature piRNA complex with the PIWIL1 homolog (Gainetdinov et al., 2018; Huang and Wong, 2021, p. 20121) (Figure 1C). Here, it is worth mentioning that, unlike the other two well-known small RNAs (miRNA and siRNA), the precursor of piRNA is a single-stranded RNA (ssRNA) rather than double-stranded RNA (dsRNA), and thus the production of mature piRNAs is independent of the enzyme Dicer, an RNase III endoribonuclease (Iwasaki et al., 2015).
1.3 TE silencing - a basic function of piRNA-PIWI complexesIn humans, transposable elements (TEs), also called “jumping genes”, and their derivatives account for 44%–48% of the genome (Autio et al., 2021; Chénais, 2022). TEs are inserted into protein-coding genes in numerous ways, causing genetic mutations and destabilizing the gene structure (Parhad and Theurkauf, 2019). Briefly, the mature piRNA complex which is exported to the nucleus from the cytoplasm, binds to complementary sites in nascent target TE transcripts and silences their expression, thus protecting the cell (Parhad and Theurkauf, 2019; Ramat and Simonelig, 2021). Additionally, piRNA complexes can silence TE transcripts in the cytoplasm and can activate, protect, and localize a wider range of other messenger RNAs (mRNAs) (Ramat and Simonelig, 2021). Most piRNAs and PIWIs gather in the “Nuage” region of the perinuclear cytoplasm, where they undergo a ping-pong cycle (Pek et al., 2012; Ge et al., 2019). The ping-pong cycle is an essential mechanism widely observed in flies, fish, and mammals, playing a critical role in piRNA amplification and the silencing of transposon transcripts (Czech and Hannon, 2016; Huang and Wong, 2021). Initially, researchers found that, in Drosophila germ cells, the 10 nucleotides at the 5′end of piRNAs that bind to Aub and Ago3, respectively, showed strong complementarity, indicating that these two classes of piRNAs are likely to interact with each other, which was later defined as the “ping-pong signature” (Brennecke et al., 2007; Li et al., 2009; Rojas-Ríos and Simonelig, 2018). For instance, during the ping-pong cycle in Drosophila germ cells, the piRNA-Aub complex binds and cleaves the target TE transcript (Rojas-Ríos and Simonelig, 2018). The cleaved fragment of the transcript with the exposed 3′ end is loaded into the Ago3 protein with the help of the Tudor-domain protein Krimper (Czech and Hannon, 2016). The Ago3 protein, guided by the RNA fragment, forms a complex with the piRNA precursor. Subsequently, the precursor is cleaved, and the fragment with the exposed 3′ end is loaded into the Aub protein, forming a new piRNA-Aub complex, aided by the Trimmer proteins (Czech and Hannon, 2016) (Figure 1D). The ping-pong cycle is conserved in other animal species, such as mouse, where the counterparts of Aub and Ago3 are MILI and MIWI2, respectively (Ernst et al., 2017; Huang and Wong, 2021), and the auxiliary proteins involved in this cycle are yet to be confirmed.
1.4 Functional implications of piRNAs and PIWIs in the soma and human diseasesAlthough initial studies in Drosophila led the researchers to believe that the piRNA pathway was limited to the germ line (Malone et al., 2009), recent studies have shown that piRNAs also function in mammalian and non-mammalian somatic tissues and stem cells. Further studies have discovered that depending on the context, for instance, in cancer cells, in addition to the piRNA-PIWI functional complex, piRNA and PIWIs can work independently of each other to control the expression of specific genes (Zhang et al., 2023).
In the current review, we explored the roles of piRNAs and PIWI proteins in stem cells other than germline, including Adult Stem Cells (ASCs), Embryonic Stem Cells (ESCs), Induced Pluripotent Stem Cells (iPSCs), etc. Additionally, we discussed the emerging roles of piRNA and PIWI proteins, both dependent and independent of each other, in regulating the expression of TE transcripts and mRNAs along with their translation in somatic cells and the development of numerous human diseases.
2 PIWIs in adult/somatic/embryonic stem cellsPlanaria is a promising animal model for studying adult stem cells and cell regeneration. Planarian adult pluripotent stem cells (aPSCs, also known as neoblasts) express three known PIWI protein homologs, PIWI-1 (SMEDWI-1), PIWI-2 (SMEDWI-2), and PIWI-3 (SMEDWI-3) (Shibata et al., 2016; Kashima et al., 2020; Li et al., 2021). Knocking down the three PIWI proteins revealed that SMEDWI-2 and SMEDWI-3 are necessary for planarian regeneration. The absence of the two PIWIs led to defects in the regenerative capacity of the planaria, and even cell death (Dattani et al., 2019), while the deficiency of SMEDWI-1 resulted in a decrease in the number of stem cells and a delay in the expansion of new cells (Allikka Parambil et al., 2024). Among the three PIWIs, SMEDWI-2 silences transposons at the epigenetic level in the nucleus (Dattani et al., 2019), and is required for homeostasis and regeneration of planarian stem cells (Almazan et al., 2018). Whereas transposons that escape nuclear silencing by SMEDWI-2 are eliminated by SMEDWI-1 and SMEDWI-3, after entering the cytoplasm, a process known as post-transcriptional silencing (Shibata et al., 2016; Li et al., 2021) (Figure 2A). SMEDWI-1 and SMEDWI-3 are the counterparts of Drosophila cytoplasmic PIWI proteins, Aub and Ago3 respectively (Sahu et al., 2017). These two PIWI proteins participate in the “ping-pong cycle” in planarian cell cytoplasm, to silence target TE transcripts and produce more piRNAs through amplification (Sahu et al., 2017; Dattani et al., 2019). As for SMEDWI-1, contrary to the earlier assumptions on its involvement as dispensable, recent studies discovered that SMEDWI-1 produces and stores piRNAs in the cell cytoplasm, binds to ribosomes, monitors poorly translated transcripts, and silences them during the pioneer round of translation (Allikka Parambil et al., 2024). Similarly, organisms such as sponges, jellyfish and planaria, carry totipotent somatic stem cells, called neoblasts (Sturm et al., 2017). Studies on the planarian Dugesia japonica revealed that DjpiwiB is essential for the differentiation of neoblasts into specialized somatic cells. Loss of DjpiwiB expression results in significant defects in the regeneration ability of neoblasts (Shibata et al., 2016; Rojas-Ríos and Simonelig, 2018) (Tables 1, 2). Additionally, investigations in the planarian Schmidtea mediterranea have shown that the PIWI-2 homologue SMEDWI-2 interacts with the heat shock protein DNAJA1, and both proteins are enriched in the planarian adult stem cells (Fernández-Taboada et al., 2011; Wang et al., 2019). Interestingly, the loss of DNAJA1 led to significant decrease in the protein levels of SMEDWI-2 in the cell cytoplasm, while the levels of SMEDWI-2 mRNA were unaltered, suggesting that DNAJA1 is likely to bind to SMEDWI-2 and maintain the stability of the latter (Wang et al., 2019) (Figure 2B). Also, DNAJA1 protects both SMEDWI-2 and piRNAs from factors like cellular environmental stress, and is essential for the maintenance of adult stem cells (Wang et al., 2019). Furthermore, studies on endodermal and ectodermal epithelial stem cells (eSCs) and i-cells of the Cnidarian Hydra have shown that it expresses two PIWI proteins, Hywi and Hyli (Nishimiya-Fujisawa and Kobayashi, 2018; Teefy et al., 2020) (Table 2). RNA immunoprecipitation studies showed that Hywi binds piRNAs and localizes to complementary sites on TE transcripts (Teefy et al., 2020). Degradome-Seq of RNA ends revealed that the 5′end of the piRNA bound to Hywi overlapped with the 5′end of the piRNA bound to Hyli by 10 bp (Juliano et al., 2014), which, together with the upregulation of TE expression brought about by the knockdown of Hywi demonstrated that Hywi and Hyli participate in the classic ping-pong cycle of the piRNA pathway to silence TE transcripts (Teefy et al., 2020). In addition, researchers have found that PIWI homolog, Hiwi, is expressed in human hematopoietic stem cells (HSCs) with CD34, and proved that it is likely to be a negative developmental regulator (Sharma et al., 2001; Rojas-Ríos and Simonelig, 2018). Subsequent studies found MIWI2 in mouse primitive hematopoietic cells and demonstrated that the loss of MIWI2 induces the differentiation of hematopoietic cells, but has little effect on the overall function of the hematopoiesis (Jacobs et al., 2013; Nolde et al., 2013; Rojas-Ríos and Simonelig, 2018).
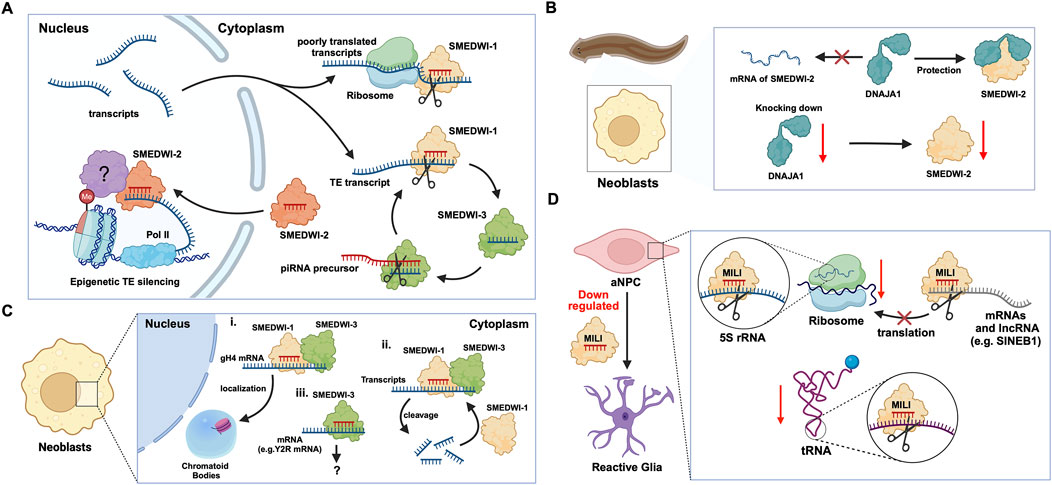
Figure 2. The functions of PIWI proteins in planarian adult pluripotent stem cells (aPSCs) and mouse adult neural progenitor cells (aNPCs). (A) In planarian aPSCs (neoblasts), TEs are mainly silenced in the nucleus. The SMEDWI-2 complex binds to TEs that are being transcribed, induces H3K9 methylation, and prevents further transcription. For other TE transcripts that successfully enter the cytoplasm, the SMEDWI-1 complex silences them through the ping-pong cycle. In addition, poorly translated transcripts are recognized and silenced by the SMEDWI-1 complex, facilitated by its interaction with the small ribosomal subunit. (B) Knockdown of DNAJA1 in neoblasts reduced the abundance of SMEDWI-2 protein but had little effect on the expression levels of SMEDWI-2 transcripts. (C) The effects of SMEDWI-1 and SMEDWI-3 on mRNA in neoblasts: (i) SMEDWI-1 and SMEDWI-3 assist germinal histone H4 (gH4) transcripts in localizing to chromatoid bodies. (ii) SMEDWI-1 and SMEDWI-3, guided by the piRNAs, cleave mRNAs that code for all the histone proteins, Traf-6, and Npk1-like proteins. The cleaved mRNAs become templates for the newly generated piRNA-SMEDWI-1 complexes. (iii) The piRNA-SMEDWI-3 complexes bind other mRNAs, such as Y2R mRNA. However, no changes were observed in the mRNA levels, and this association’s outcome is yet to be determined (denoted as ?). (D) The piRNA-MILI complex is involved in the preservation of mouse aNPC pluripotency. (left) Artificial knockout of MILI causes aNPCs to differentiate into unhealthy astrocytes, also referred to, as reactive glia. (right) The piRNA-MILI complex inhibits protein synthesis in aNPCs by silencing tRNA, 5S rRNA, SINEB1 and mRNAs encoding ribosomal proteins, thereby slowing down their differentiation.
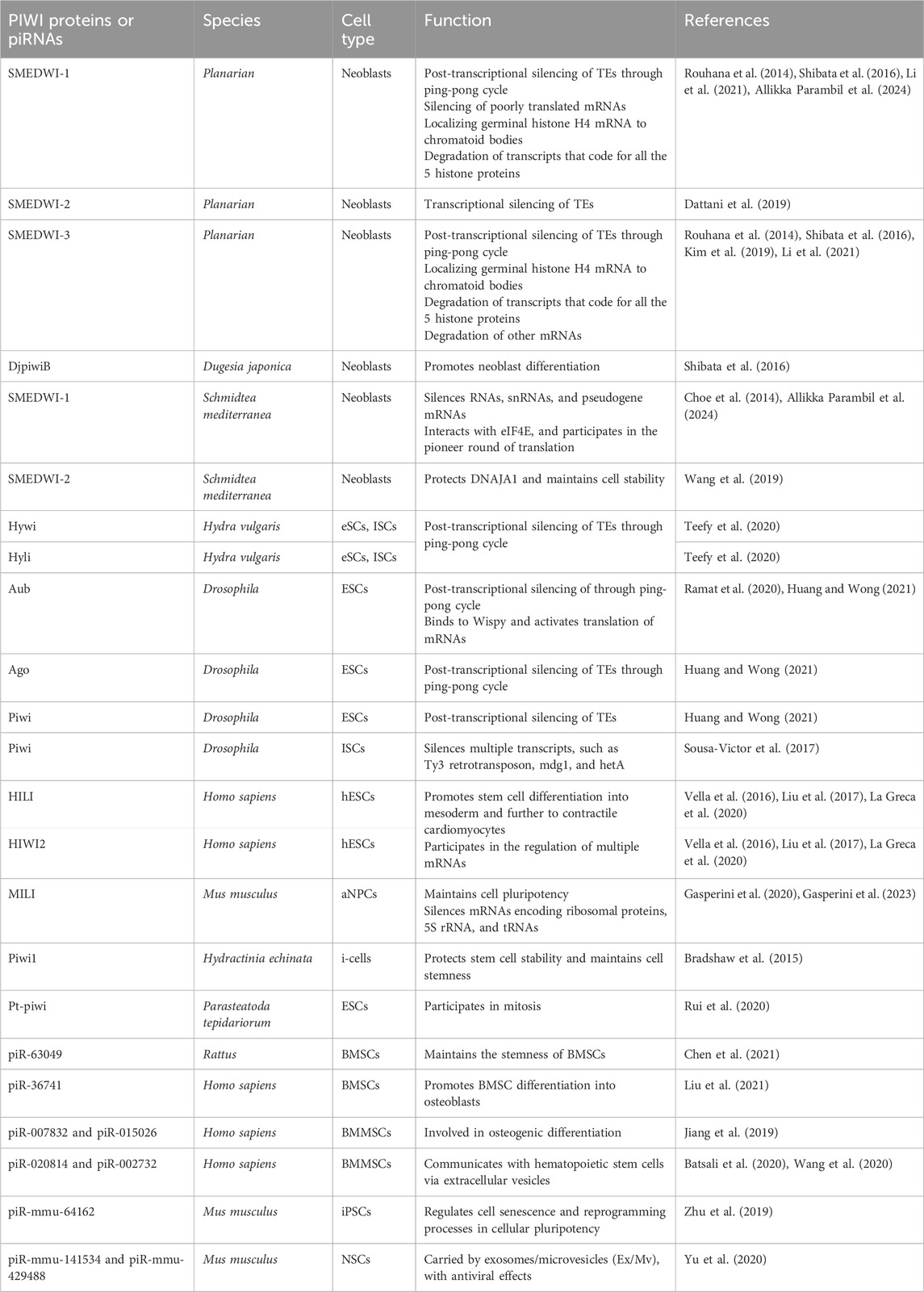
Table 2. piRNAs, PIWIs and their roles in adult stem cells.
2.1 piRNA-PIWI-mediated regulation of mRNAsIn planarian neoblasts, SMEDWI-1 and SMEDWI-3 were reported to be crucial for the localization of germinal histone H4 (gH4) mRNA to chromatoid bodies (CB), a type of ribonucleoprotein (RNP) granules located in the perinuclear cytoplasm, and these PIWIs are indispensable for the formation of CBs(Rouhana et al., 2014). Also, recent studies have found that the piRNA- SMEDWI-1 complex binds to and regulates non-polyadenylated mRNAs, including stem-loop transcripts of all five histones during the S phase of the cell cycle (Allikka Parambil et al., 2024). Interestingly, during the differentiation of neoblasts, both CB and neoblast histone transcripts are degraded. More than 50 piRNAs which match the coding sequences of the transcripts of five histones respectfully have been identified. These piRNAs are likely to be the derivatives of a cleaved histone mRNA, and may therefore serve as potential candidates for guiding PIWI cleavage (Rouhana et al., 2014). Yet, it remains to be elucidated which specific PIWI proteins are involved in the degradation of histone transcripts. Additional work has demonstrated that SMEDWI-3 is involved in the degradation of a large number of mRNAs, including the transcripts of Traf-6 and Npk1-like genes, in neoblasts. Traf-6 is closely linked to the differentiation and proliferation of planarian stem cells, while the Npk1-like gene has not been studied in animals yet. At the same time, for other mRNAs, such as the transcripts of Y2R and TEX2, the piRNA-SMEDWI-3 complex binds to them but does not yield any change in their abundance, suggesting a translational control of these transcripts (Figure 2C; Table 2) or the RNA stabilization by the bound PIWIs, to maintain their function in the regulation of cell cycle (Kim et al., 2019). Likewise, in human embryonic stem cell (hESC) lines (H1, H9), during the differentiation of these pluripotent cells, the expression of HILI mRNA was significantly downregulated, while that of HIWI2 was upregulated (Liu et al., 2017; La Greca et al., 2020). In addition, small RNA-seq data showed that multiple piRNAs, including piR-4403262 and piR-4424378, may be involved in the regulation of numerous mRNAs in cardiomyocytes, including transcripts of MALAT1, TTN, and PLN (La Greca et al., 2020). Additionally, studies have shown that piRNAs promote the differentiation of cardiac progenitor cells and the regeneration of cardiac cells (Rajan et al., 2014). There are hundreds of upregulated or downregulated piRNAs involved in this process, including piR-30444, piR-46538 as over-expressed, and piR-30136, piR-30368 down-expressed (Vella et al., 2016). These upregulated piRNAs silence LINE-1 and promote the differentiation of cardiac progenitor cells and the regeneration of cardiac cells by activating the AKT signalling pathway (Vella et al., 2016). Furthermore, it is evident that in the hippocampus of newborn mice, the adult neural progenitor cells (aNPCs) are rich in PIWIL2/MILI expression, and more than 570,000 putative piRNAs were identified from small RNA libraries, including one homologous to the human piR-61648. The reads of these putative piRNAs were significantly reduced, as aNPCs differentiated into neurons (Gasperini et al., 2020; Gasperini et al., 2023). Surprisingly, through in silico analysis, researchers discovered that repeats in 5S rRNA and tRNAs are important targets of piRNAs in aNPCs, in addition to the known targets such as mRNAs encoding ribosomal proteins, and transcripts including SINEB1 (Gasperini et al., 2020) (Figure 2D). By way of regulation, these piRNAs are anticipated to inhibit ribosome assembly, thereby reducing the rate of protein synthesis in aNPCs, and maintaining cell stemness (Gasperini et al., 2020). Moreover, the lack of MILI and piRNAs results in damage to neurogenesis, and forms reactive glia, which are commonly produced after damage to the nervous system, with its appearance often accompanied by inflammation and aging (Gasperini et al., 2023) (Table 2). Similarly, in the early Drosophila embryo, Aub was found to directly interact with the protein Wispy, a non-canonical poly(A) polymerase that polyadenylates the long poly(A) tails of maternal mRNAs related to reproduction in the early embryo, thereby protecting these mRNAs(Dufourt et al., 2017). The piRNA-Aub-dependent stabilization of mRNAs has already been shown to be necessary for protecting nanos in the early Drosophila embryo, which is considered as a germline stemness marker (Ramat and Simonelig, 2021; Rinkevich et al., 2022). Furthermore, maternal piRNA- Aub directly recruits the poly(A)-binding protein (PABP) that protects mRNA through binding to the poly(A) tail (Ramat and Simonelig, 2021). Here, Aub interacts with eIF3d, a subunit of eukaryotic initiation factor 3 complex (eIF3), initiating the translation of the target mRNAs including Nanos (Figure 3A). Nanos is closely related to the development of the germline, thereby bypassing the classic but rate-limiting protein synthesis mechanism, which is activated by eukaryotic initiation factor 4E (eIF4E) binding to the mRNA 5′cap (Ramat et al., 2020; Ramat and Simonelig, 2021).
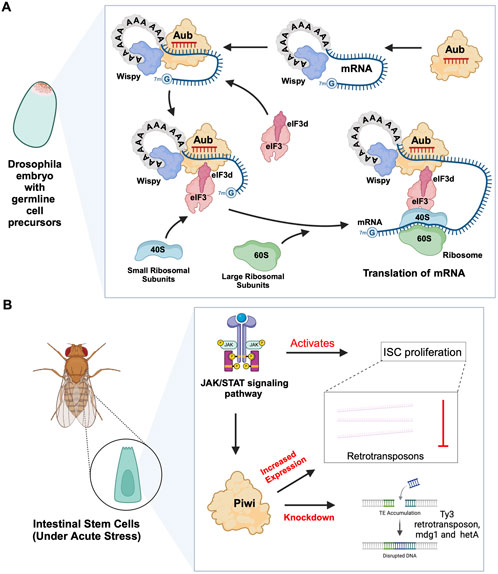
Figure 3. piRNA-PIWI-mediated mRNA regulation in Drosophila adult stem cells. (A) Aub activating mRNA translation in the early Drosophila embryo: The piRNA-Aub complex binds to complementary regions within mRNAs bound by the poly(A)-binding protein Wispy. When the mRNA needs to be activated, the Aub complex interacts with Wispy, and recruits eIF3d subunit, which is part of eIF3 complex, along with the 40S ribosomal subunit, thereby initiating the process of translation. (B) Role of JAK/STAT pathway and PIWI proteins in Drosophila ISCs: The JAK/STAT pathway activates ISC proliferation and induces the expression of Drosophila Piwi protein, which silences transposons and ensures gene stability. Knocking down Piwi led to the accumulation of multiple TE transcripts, including Ty3 retrotransposon, mdg1 and hetA.
2.2 piRNA-independent functions of PIWIsPrevious studies on Drosophila intestinal stem cells (ISCs) show that JAK/STAT signalling can activate ISC proliferation (Ayyaz and Jasper, 2013). Under acute stress, the JAK/STAT pathway not only induces ISC proliferation but also induces the increased expression of Drosophila Piwi protein, thereby silencing retrotransposons and ensuring gene stability (Sousa-Victor et al., 2017; Rojas-Ríos and Simonelig, 2018). Knocking down piwi led to the accumulation of multiple TE transcripts, such as Ty3 retrotransposon, mdg1 and hetA (Figure 3B; Table 2), while in contrast, the loss of Aub and Ago3, which belong to the same PIWI protein family, did not affect TE silencing (Sousa-Victor et al., 2017). It is still uncertain whether the functions of these PIWI proteins depend on piRNAs(Sousa-Victor et al., 2017; Tang et al., 2023). It is worth mentioning that recent small RNA-seq data from the Drosophila intestine also failed to provide evidence of piRNAs’ existence (Siudeja et al., 2021). Thus, provides for the hypothesis that PIWI proteins may act alone in Drosophila ISCs, independent of piRNA sequences. Moreover, the STAT signalling-dependent Piwi protein activation was detected in enteroblasts, the ISC daughter cells with pluripotency, further strengthening the case for piRNA-independent PIWI function in ISCs(Sousa-Victor et al., 2017). Likewise, under disease conditions, namely in glioblastoma (GBM), PIWIL1 plays a significant role by promoting the self-renewal of glioma stem cells (GSCs). Overexpression of PIWIL1 has been consistently observed in these GSCs, contributing to their maintenance and tumorigenic potential (Huang et al., 2021). However, knockdown experiments targeting PIWIL1 have revealed a contrasting effect. The knockdown of PIWIL1 triggers an increase in tumor suppressors BTG2 and FBXW7, leading to the destabilization of the oncogene c-Myc, which in turn diminishes the proliferation and self-renewal capabilities of GSCs (Figure 4A). This effect is mediated by the enhanced stability and expression of BTG2 and FBXW7 mRNAs, which are crucial for inhibiting cell cycle progression and facilitating the degradation of oncogenic proteins like c-Myc. Consequently, reducing key stem cell factors such as Olig2 and Nestin hampers the maintenance of GSCs. These findings suggest that PIWIL1 contributes to glioblastoma growth through its regulatory effects on mRNAs. While these observations provide insights into PIWIL1’s function in mRNA regulation, further studies are needed to clarify whether these effects occur independently of piRNAs or involve indirect mechanisms (Huang et al., 2021). Also, in studying the expression of piRNA pathway genes in cancer, Genzor et al. (2019) investigated whether these genes could restore functional piRNA silencing in non-germline cells. They found that while genes like PIWIL1, PIWIL2, PLD6 (Zucchini), and DDX4 (VASA) were expressed in some cancer cell lines, their expression alone was not enough to form functional piRNA-PIWI silencing complexes (piRISCs). For example, in COLO205 cells, which have high levels of PIWIL1 but lack DDX4 and other key components, small RNAs bound to PIWIL1 did not show the features of true piRNAs. Instead, they resembled other RNA fragments, such as microRNAs, and did not exhibit piRNA activity (Genzor et al., 2019). Additionally, knocking down or deleting PIWIL1 did not impact transposon transcript levels, indicating that the piRNA pathway was not functional in this setting. These findings highlight the importance of careful analysis to confirm true piRNA activity and avoid misinterpreting aberrant gene expression in cancer.
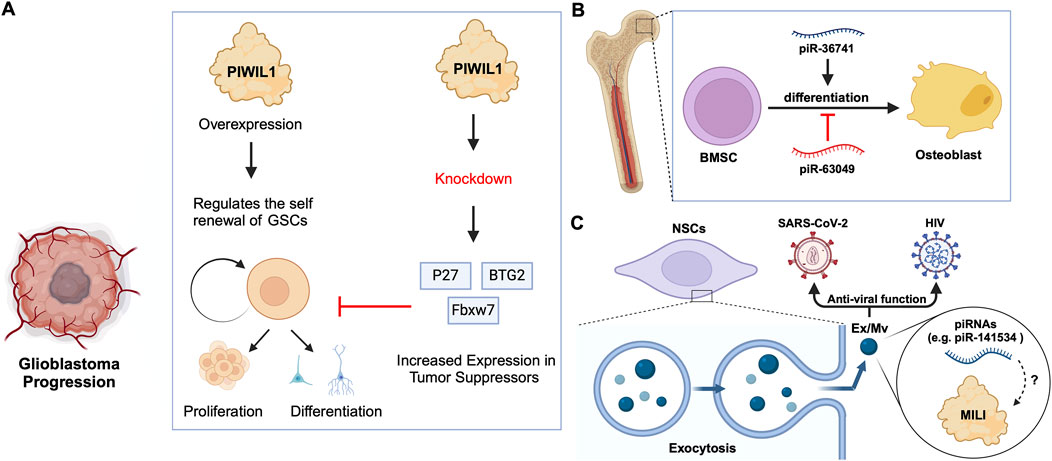
Figure 4. piRNA-specific and independent function of PIWIs in stem cell differentiation. (A) PIWIL1 overexpression in glioblastoma stem cells promotes self-renewal and tumorigenic potential. Knockdown of PIWIL1 upregulates tumor suppressors, namely BTG2 and FBXW7, inhibiting GSC self-renewal and proliferation, while promoting differentiation and senescence. (B) piRNAs in the differentiation of BMSCs: piR-36741 promotes the differentiation of BMSCs into osteoblasts, while piR-63049 inhibits this process. (C) piRNAs in exosomes/microvesicles have anti-viral roles. Mouse NSCs excrete Ex/Mvs carrying piRNAs out of the cells via exocytosis. These Ex/Mvs specific piRNAs bind and degrade target viral RNAs of HIV and SARS-Cov-2. MILI was identified as cargo in Ex/Mvs, and however, its role in the piRNA-guided antiviral functions is yet to be demonstrated (denoted as ?).
2.3 Roles of specific piRNAsBone marrow stromal cells (BMSCs) include multipotent cells such as osteochondral and adipocyte progenitors (Gao et al., 2022). Through studies on BMSCs in rats and other mammals, researchers found that piRNAs are crucial for osteoblast differentiation. For instance, the piRNA, piR-63049 plays a significant role in maintaining the stemness of BMSCs(Chen et al., 2021). Meanwhile, other piRNAs, such as piR-016735, were found in exosomes derived from BMSCs, and the piRNA overexpression inhibits the differentiation of BMSCs into osteoblasts (Wang et al., 2020; Chen et al., 2021). Correspondingly, studies on human BMSCs have revealed that the expression of another piRNA, piR-36741, promotes BMSC differentiation into osteoblasts (Liu et al., 2021; Mishra et al., 2023) (Figure 4B; Table 2). Another study on human dental apical papilla stem cells (SCAP) and bone marrow mesenchymal stem cells (BMMSCs) showed that piRNAs such as piR-007832 and piR-015026 are highly expressed in BMMSCs, and the target genes of these piRNAs are enriched in pathways such as p53 signalling and the cascade associated with Epstein-Barr virus infection (Wang et al., 2020), likely involved in osteogenic differentiation (Jiang et al., 2019). Whereas, the piRNAs reported in SCAP are associated with MAPK signalling, citrate cycle, and Ras signalling pathways (Wang et al., 2020), among which, the MAPK signalling was closely related to the odonto-/osteogenic differentiation of SCAP (Wang et al., 2018). Interestingly, BMMSCs are believed to communicate with hematopoietic stem cells (HSCs) via extracellular vesicles (EVs) (Batsali et al., 2020) (Table 2). By sequencing small RNAs in human EVs, De Luca, L. et al. found at least two piRNAs, namely piR-020814 and piR-002732 affected the expression of numerous proteins including MPO, SLC2A5, SLAMF8, and CYP1B1(De Luca et al., 2016). These proteins have various functions such as antibacterial activity, facilitation of fructose transport, and are involved in adaptive and innate immune responses, and participate in the oxidative metabolism of xenobiotics. Likewise, induced pluripotent stem (iPS) cells from mice express at least one piRNA, piR-mmu-64162, which regulates the expression of proteins, such as Ccnd1 and Hipk1, that promote cellular reprogramming and resist cell senescence (Zhu et al., 2019). However, previous triple knockout of PIWI proteins (MILI, MIWI, MIWI2) in mouse iPS cells showed that the lack of PIWI proteins did not affect the expansion and gene expression of iPSCs(Cheng et al., 2014), raising the possibility that piRNAs function independently of PIWIs in iPSCs, which requires further studies to strengthen the argument. Similarly, murine Neural Stem Cells (NSCs) were reported to attack and neutralize viruses by releasing exosomes/microvesicles (Ex/Mv) that are enriched in approximately 150 piRNAs, including piR-mmu-141534 and piR-mmu-429488, with potential antiviral capabilities, targeting the genomes of HIV, lentiviruses and SARS-CoV-2 (Yu et al., 2020) (Figure 4C; Table 2). Furthermore, the authors of the study detected PIWIL2/MILI in the NSC Ex/Mv, however, the specific roles of this protein and how or whether it regulates specific piRNA function in attributing antiviral functions of those NSC Ex/Mv remains to be determined (Yu et al., 2020).
3 Emerging model systems for the study of piRNA and PIWI functions in adult/somatic stem cellsIn addition to the effects on mRNAs and TE transcripts, recent studies on neoblasts of the planarian Schmidtea mediterranea have shown that SMEDWI-1-bound piRNAs recognize and silence other non-coding RNAs, including rRNA, snRNA, and pseudogene mRNAs(Allikka Parambil et al., 2024) (Figure 5A). SMEDWI-1 was found to physically interact with eIF4A3, which is involved in the RNA unwinding, during the pioneer round of mRNA translation, suggesting the involvement of SMEDWI-1 in this process (Choe et al., 2014; Allikka Parambil et al., 2024) (Table 2). Furthermore, in planaria, Nb2 is a subtype of clonogenic neoblasts with pluripotent cell characteristics which have a high expression of PIWI-1 (Zeng et al., 2018). Here, the high levels of PIWI-1 are closely related to active cell division, as in most cases, PIWI-1 has long been considered an essential protein for the pluripotency of neoblasts (Srivastava et al., 2014; Zeng et al., 2018; Raz et al., 2021). TSPAN-1 is a surface protein on the Nb2 cell membrane that can induce stem cell mobilization and regeneration (Zeng et al., 2018). TSPAN-1+ cells exhibit pluripotency and can clone themselves to form new neoblasts, and also produce a variety of progenitor cells, such as epidermal, neural, and muscle progenitor cells (Molina and Cebrià, 2021). The TSPAN-1 protein is difficult to detect in cells at steady state, but interestingly, it is highly expressed in cells with enhanced expression of PIWI-1, which in turn is closely related to active cell division (Zeng et al., 2018; Pearson, 2022). Yet, it is still unknown how TSPAN-1 levels correlate with PIWI-1 and if any, whether PIWI-1 directly controls the abundance and/or translation of TSPAN-1 mRNA, guided or not, by specific piRNAs (Figure 5B). Similarly, the analysis of the genomic library of nemertean ribbon worm Lineus sanguineus identified three PIWI protein homologs, Ls-piwi1, Ls-piwi2 and Ls-piwi3, which is consistent with the data obtained from Notospermus geniculatus in the same phylum, Nemertea (Xu and Sun, 2020) (Table 1). Although there is no clear evidence that this organism has congenital stem cells, its somatic cell population likely contain other pluripotent cells (Zattara et al., 2019). Among the PIWIs reported in L. sanguineus, Ls-piwi1 is actively expressed during stem cell self-renewal, but downregulated drastically to the end of the process of differentiation, while Ls-piwi3 is only detected during posterior regeneration, and the expression of Ls-piwi2 is very weak at all stages (Xu and Sun, 2020). Moreover, the latest study on neoblasts of the acoel worm Hofstenia miamia showed that, in cells with high expression of a variant of histone H3 (H3.3), the expression level of piwi-1 was the highest among all neoblast subpopulations in the post-embryonic and regenerative periods (Hulett et al., 2023). This histone variant is believed to be related to the differentiation of muscle, nerve, and epidermal cells. Moreover, in neoblasts with high expression of piwi-1, differences in transcription factors (TFs) were reported to affect the direction of differentiation (Hulett et al., 2022). However, whether H3.3 or TFs directly alter the expression and/or the function of piwi-1 and its interacting partners with implications in co- or post-transcriptional gene silencing has not been elucidated (Figure 5C). Furthermore, the phylum Cnidaria includes aquatic organisms such as corals, sea anemones, jellyfish, and hydroids. One of the common adult stem cells in these organisms is called interstitial cells (i-cells). Studies on the Cnidaria Hydractinia symbiolongicarpus have shown that Piwi1 is active in i-cells, but its expression is reduced as i-cells differentiate into somatic cells (Varley et al., 2022). Interestingly, the i-cells expressing Piwi1 differentiate toward germ cells, after induction by Tfap2 (DuBuc et al., 2020). However, the molecular details governing this process remain unknown (Figure 5D). Further experimentation on another Cnidaria, Hydractinia echinata has shown that knocking down Piwi1 in i-cells impaired i-cell differentiation, thereby inhibiting the regeneration ability of H. echinate, after damage (Bradshaw et al., 2015; Rojas-Ríos and Simonelig, 2018). Likewise, in marine invertebrates, namely, the Ctenophore Mnemiopsis, researchers have found that the regions where MlPiwi1 is highly expressed, overlap with the regions with putative stem cell gene expression (Reitzel et al., 2016) (Table 1). These findings are consistent with previous reports on another Ctenophore, Pleurobrachia, where the piwi gene is actively expressed in regions with high mitotic activity (Alié et al., 2011). Beyond the aforementioned examples, studies on the spider Parasteatoda tepidariorum have shown that Pt-piwi is ubiquitously expressed in the early stages of spider embryonic development, and is essential for mitosis in early embryos, while RNAi treatment of Pt-piwi causes embryonic death (Schwager et al., 2015) (Table 1). Most embryonic tissues maintained high expression of Pt-piwi until the late embryonic stage when Pt-piwi accumulated in the formation area of primordial germ cells (PGCs) (Schwager et al., 2015) – the precise characteristic of PIWI proteins that made them a widely used potential biomarker for PGCs(Rui et al., 2020).
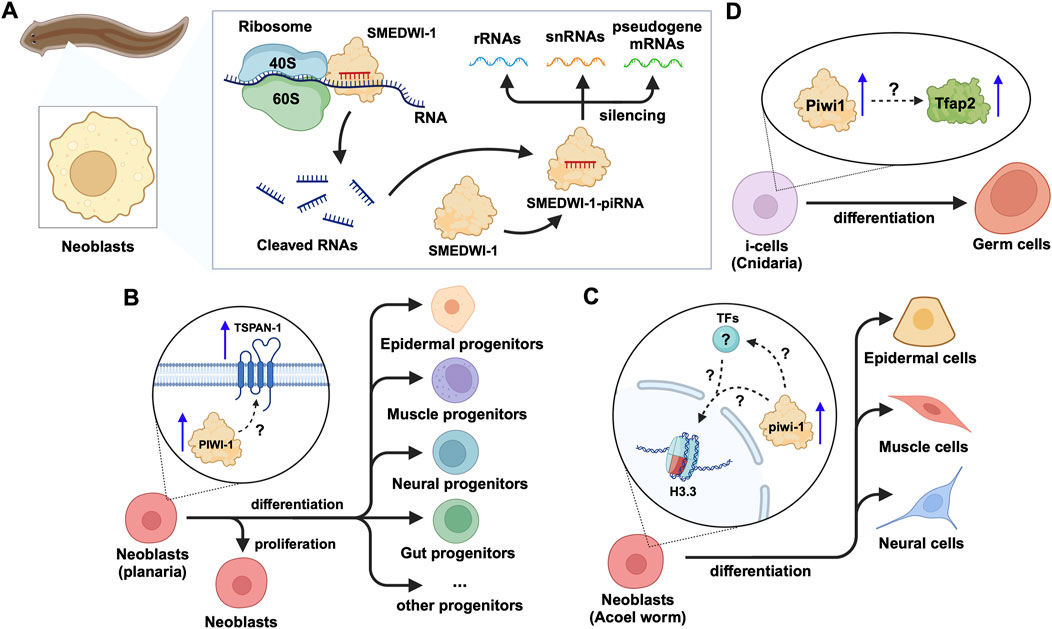
Figure 5. piRNA and PIWI functions in emerging models of adult stem cells in animals. (A) In planarian neoblasts, the SMEDWI-1-piRNA complex binds to the small ribosome subunit to cleave rRNAs, snRNAs, or pseudogene mRNAs. The cleaved RNA fragments will form new SMEDWI-1-piRNA complexes and continue to participate in target RNA cleavage. (B) In actively dividing neoblasts, the expression of PIWI-1 and TSPAN-1 has been detected to increase simultaneously. However, the connection between them has not been fully established in terms of their expression changes and the downstream effects on neoblast differentiation into a variety of progenitor cells. (C) High piwi-1 expression was detected during the differentiation of neoblasts expressing the histone variant H3.3, into epidermal, muscle and neural cells. The likely interplay between piwi-1, H3.3 and TFs involved in the process, is yet to be explored (denoted as ?). (D) In Cnidaria, Tfap2 and Piwi-1 jointly induce i-cells to differentiate into germ cells, with little information known in terms of how Piwi-1 controls Tfap2 expression.
4 piRNA-dependent and independent functions of PIWIs in somatic tissuesSince the identification of piRNAs as an emerging class of small RNAs, they have substantially enriched our understanding of gene regulatory mechanisms, by way of their crucial functions, mediated by PIWI proteins, in germline processes, controlling the expression of retrotransposons (Grivna et al., 2006; Houwing et al., 2007; Loubalova et al., 2023). However, in 2015, Haifan et al. acknowledged new research exploring the roles of PIWI proteins and piRNAs in somatic cells (Ross et al., 2014). After initial investigations into the somatic role of the piRNA pathway in the ovarian cells of Drosophila, recent insights have revealed its functional relevance in various other tissues, such as the brain of Drosophila and mice (Nandi et al., 2016; Perrat et al., 2013). Additionally, a new dimension, that is, the function of PIWIs can either be dependent or independent of piRNAs, has gained traction over the years, with emerging reports constantly supporting the notion, pending in-depth validation. Thus far, the primary emphasis in somatic cell research has centred on unravelling the involvement of piRNA and PIWI proteins in diseases like cancer. However, recent studies have revealed their involvement in other human diseases such as respiratory, cardiovascular, and neurodegenerative disorders. Previously, it was believed that PIWI protein functionality relied exclusively on piRNAs. As recent research has demonstrated the non-canonical functions of PIWIs, namely their roles independent of piRNAs(Lin et al., 2020). We will provide a comparable and contrasting view of piRNA functions and PIWI proteins in TE silencing and mRNA regulation in the soma.
4.1 piRNA-dependent function of PIWIsPIWI proteins form a complex with piRNAs, namely piRNA-induced silencing complex (piRISC), to mediate both transcriptional and post-transcriptional gene silencing (Loubalova et al., 2023). Not all PIWI proteins operate similarly or at the same level in the regulation of gene expression. While MILI/PIWIL2 and MIWI/PIWIL1 are involved at the post-transcriptional level, MIWI2/PIWIL4 primarily engages in transcriptional gene silencing (Loubalova et al., 2023). Here, we will specifically discuss the role of the piRNA-PIWI complexes in the regulation of mRNAs and retrotransposon silencing at the post-transcriptional level.
4.1.1 mRNA regulationMuch has been established in the animal germline, where, in C. elegans, piRNAs target the germline transcriptome through incomplete base-pairing, thereby regulating genes transgenerationally (Wang X. et al., 2023). Also, mouse pachytene piRNAs, mostly unrelated to TE sequences, loaded onto MIWI, promote mRNA deadenylation and decay by recruiting chromatin assembly factor 1 (CAF1), and they can also direct MIWI to cleave mRNAs in a siRNA-like manner (Gou et al., 2014; Wang X. et al., 2023). Additionally, in Drosophila germline, specifically, in germline stem cells, it was shown that Aub/piRNAs control the expression levels of cbl (Rojas-Ríos et al., 2017) and dunce mRNAs(Ma et al., 2017). However, in somatic cells, piRNAs and PIWI proteins are not widely expressed and are primarily associated with various diseases, which will be discussed later in this review. In D. melanogaster, piRNA-loaded Aub destabilizes maternal mRNAs through endonucleolytic cleavage or by recruiting the CCR4–NOT complex consisting of exonucleases (Rouget et al., 2010; Barckmann et al., 2015; Lee et al., 2017; Wang X. et al., 2023). Further, in C. elegans, the PIWI protein PRG-1 is expressed in adult mechanosensory neurons and limits axon regeneration after injury, through piRNA-dependent post-transcriptional mRNA regulation. Here, PRG-1, guided by piRNAs, cleaves and represses target mRNAs including prde-1 and prg-1. PRDE-1 facilitates piRNA precursor transcription and autonomously inhibits axon regrowth in C. elegans, while PRG-1 uses piRNA-guided slicer activity to repress mRNAs, limiting axon regeneration and ensuring germline stability (Kim et al., 2018). Additionally, in 2018, Balaratnam et al. demonstrated the post-transcriptional regulation of the FTH1 gene by piRNAs in somatic cells (Balaratnam et al., 2018). FTH1 is elevated in a variety of cancers and has antioxidant properties (Shpyleva et al., 2011; Alkhateeb and Connor, 2013). They showed that piR-FTH1 downregulates Fth1 mRNA in triple-negative breast cancer (TNBC) cells, namely, MDA-MB-231, which indicates a potential therapeutic strategy for reducing FTH1 levels in cancer cells (Balaratnam et al., 2018). Furthermore, the study revealed that two PIWI proteins, HILI and HIWI2 are involved in mediating piR-FTH1-guided post-transcriptional regulation of the FTH1 gene. FTH1 encodes a protein which is part of the ferritin complex, responsible for iron storage and regulation in the body. It plays a crucial role in managing iron homeostasis and protecting cells from oxidative damage (Balaratnam et al., 2018).
4.1.2 Retrotransposon silencingRepetitive sequences, including TEs, constitute a significant portion of mammalian genomes and are found in nearly all organisms, including D. melanogaster (Curcio and Derbyshire, 2003; De Koning et al., 2011; Huang et al., 2012; Ernst et al., 2017). Retrotransposons, classified as Class I TEs, utilize a “copy and paste” mechanism, which involves RNA intermediates (Ho et al., 2024). In the germline of D. melanogaster, Aub and Ago3 proteins post-transcriptionally silence retrotransposon transcripts, guided by antisense piRNA sequences (Senti et al., 2015; Wang et al., 2015). Also, Watanabe et al., in 2015, revealed that piRNAs originating from pseudogenes and retrotransposons govern the expression of both functional cognate mRNA transcripts, and RNAs harbouring retrotransposon sequences. This regulatory mechanism extends to L1 retrotransposons, thereby modulating their activity, and thus safeguarding genomic stability throughout spermatogenesis (Watanabe et al., 2015). In somatic tissues, the repression of retrotransposons involves a piRNA pathway similar to the one in germ cells (Lewis et al., 2017). Briefly, cytoplasmic PIWI proteins, namely, Aub and Ago3 in Drosophila, and, MIWI and MILI in mice, cleave TE transcripts and help amplify piRNAs in the germline (Wang X. et al., 2023). However, nuclear PIWI proteins, specifically, Piwi in Drosophila, and MIWI2 in mice, bind to nascent RNAs at TE sites and recruit other proteins to silence transcription, without degrading the transcripts. Key proteins, including Maelstrom (Mael) and Panoramix (Panx), are crucial in this process (Sienski et al., 2012; Wang X. et al., 2023). Mael interacts with the Brahma complex to reduce TE transcription in somatic cells, while Panx induces transcriptional silencing and heterochromatin formation by forming complexes with Nxf2 and Nxt1 (Onishi et al., 2020). The SUMO ligase Su(var)2–10 further connects Piwi complexes to the machinery that silences chromatin, enhancing TE repression (Ninova et al., 2020b; Ninova et al., 2020a). These interactions ensure effective TE silencing in somatic tissues, demonstrating the piRNA pathway’s essential role in protecting the genome beyond germ cells.
5 piRNA-PIWIs in agingThe role of piRNA-PIWIs in the aging process has not been widely discussed. Recent insights in C. elegans have highlighted that transposable elements (TEs) contribute significantly to aging by promoting genomic instability, with their activity increasing as the organism ages due to epigenetic changes, such as the accumulation of N6-adenine methylation at active TE loci (Sturm et al., 2023). The piRNA- PIWI pathway, essential in germline cells for silencing TEs, was shown to enhance lifespan when ectopically expressed in somatic cells, likely by suppressing TEs and reducing genomic instability. Downregulation of active TEs, such as Tc1 and Tc3, extended lifespan in C. elegans, while elevated TE activity was associated with somatic decline, supporting a direct causal link between TE mobilization and aging (Sturm et al., 2023). Moreover, these findings align with recent insights that piRNA-PIWI influences longevity by modulating Hedgehog signalling after mating-induced germline hyperactivity (Shi and Murphy, 2023). The downregulation of piRNAs leads to the activation of Hedgehog-like ligands, which communicate germline status to somatic cells, resulting in somatic collapse and reduced lifespan (Shi and Murphy, 2023) (Figure 6). Wet age-related macular degeneration (wAMD) is a major cause of blindness in older adults in industrialized countries (Muraleva et al., 2019). PIWIL4, also referred to as HIWI2 in humans and MIWI2 in mice is involved in choroidal neovascularization (CNV), suggesting a critical role for piRNA-PIWIs in the pathological processes underlying wAMD (Ren et al., 2023). It plays a key role by controlling VEGF-driven angiogenesis through its interaction with specific piRNAs. Studies using laser-induced CNV models in mice showed increased PIWIL4 expression in retinal pigment epithelium (RPE)-choroid-sclera complexes and choroidal endothelial cells (MCECs). Experimental evidence demonstrated that reducing PIWIL4 levels with shRNA lentiviral and adeno-associated virus (AAV) systems lowered CNV lesion areas and reduced abnormal blood vessel growth in vivo. In vitro silencing of PIWIL4 blocked VEGF-induced cell growth, migration, and tube formation, while also lowering VEGF secretion and VEGFR2 activity, showing its importance in VEGF-related pathways. High-throughput sequencing identified several piRNAs, including piR-mmu-72603, piR-mmu-671578, and piR-mmu-7577,132, as highly expressed in CNV tissues (Ren et al., 2023). These piRNAs, likely working with PIWIL4, were linked to blood vessel growth and repair through biological pathway analyses. The co-localization of PIWIL4 with areas of neovascularization further highlights its role in abnormal blood vessel growth. Together, these findings suggest that the PIWIL4-piRNA system is central to the development of wAMD and may serve as a target for new treatments. Additionally, the presence of piRNAs in eye fluids suggests their potential as non-invasive biomarkers, opening new possibilities for precise diagnosis and treatment of wAMD (Ren et al., 2023).
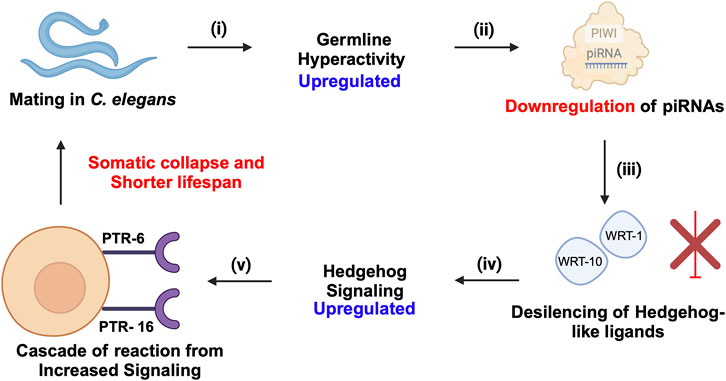
Figure 6. piRNA-PIWI-mediated regulation of mating-induced germline hyperactivity and somatic collapse in the aging of C. elegans. (i) Mating initiates hyperactivity in the germline. (ii) This germline hyperactivity leads to the downregulation of piRNAs. (iii) The downregulation of piRNAs results in the de-silencing and activation of Hedgehog-like ligands, such as wrt-1 and wrt-10. (iv) The activation of these Hedgehog-like ligands enhances Hedgehog signalling, which impacts somatic cells through specific receptors, PTR-6 and PTR-16. (v) The increased Hedgehog signalling in somatic cells triggers a cascade of events leading to somatic collapse, characterized by a decline in somatic cell function and health, ultimately accelerating aging and reducing lifespan.
6 piRNAs and PIWI proteins in human diseasesThe independent roles of PIWI proteins outside their traditional association with piRNAs have been explored mainly in the context of human diseases. The expression and function of PIWIs and potential piRNAs along with the downstream targets responsible for the disease phenotype are detailed in Table 3.
Comments (0)