Various types of blood cells, such as lymphoid and myeloid cells, are produced from hematopoietic stem cells (HSCs) in the bone marrow, which is not the site of HSC development. During mouse embryogenesis, HSC development begins with endothelial cell differentiation. Some endothelial cells undergo an endothelial-to-hematopoietic transition (EHT), giving rise to HSC-precursors (pre-HSCs) (Taoudi et al., 2008; Zhou et al., 2016). The pre-HSCs then migrate to the fetal liver and mature into HSCs, which ultimately move to the bone marrow and maintain lifelong hematopoiesis (Dzierzak and Speck, 2008; Orkin and Zon, 2008).
HSCs arise from endothelial cells in the aorta-gonad-mesonephros (AGM) region (Muller et al., 1994; Cumano et al., 1996; Medvinsky and Dzierzak, 1996; de Bruijn et al., 2002; Zovein et al., 2008). Although the HSC developmental process appears simple, there are branching points at each differentiation stage that determine the cell fate. Most endothelial cells engage in the formation of blood vessels, but a small proportion of these cells differentiate into hemogenic endothelial cells. These hemogenic endothelial cells undergo an EHT and differentiate into pre-HSCs as well as hematopoietic progenitor cell precursors (pre-HPCs), which enter into processes that lead to the formation of intra-aortic hematopoietic clusters (IAHCs) in the dorsal aorta of the AGM region between the embryonic day (E) 9.5 and E11.5 (Zovein et al., 2008; Chen et al., 2009; Boisset et al., 2010; Yokomizo and Dzierzak, 2010; Boisset et al., 2015). Here, we focus on the transcription factors and their target genes, and discuss hemogenic endothelium development, EHT, and IAHC formation. We also summarize the signaling molecules that are expressed during HSC development and the timing of their expression, focusing primarily on findings obtained from in vitro culture studies.
Developmental pathway of HSCsDevelopment of hemogenic endotheliumThe first HSCs were observed in the AGM region during midgestation (Muller et al., 1994; Medvinsky and Dzierzak, 1996), and were shown to originate from IAHCs derived from endothelial cells in the innermost layer of the AGM region (Jaffredo et al., 1998; Zovein et al., 2008; Boisset et al., 2010). Whereas most endothelial cells eventually contribute to blood vessel formation, some differentiate into hemogenic endothelium, which undergoes an EHT to generate IAHCs. Amongst several transcription factors, RUNX1 has been shown to be a key factor during this sequence. RUNX1 is important for hematopoietic development and is lethal in knockout mice around E12.5 due to extensive hemorrhages (Okuda et al., 1996). North et al. showed the generation of HSCs from Runx1-expressing cells (North et al., 2002), with RUNX1 contributing to HSC production via an enhancer regulated by GATA/ETS/SCL (Nottingham et al., 2007). The activation of this enhancer was initiated in the endothelial cell layer, suggesting that hemogenic endothelium development likely occurs in the endothelial cells (Swiers et al., 2013). Indeed, when Runx1 is deleted in cells expressing the endothelial cell markers Cdh5 or Tie2, HSCs are not generated (Li et al., 2006; Chen et al., 2009), indicating that endothelial cells are the origin of HSCs and that RUNX1 contributes to their developmental process.
RUNX1 functions as a transcription factor by forming a dimer with CBFB; thus, Cbfb-deficient mice exhibit the same phenotype of definitive hematopoietic deficiency as Runx1-deficient mice. Chen et al. attempted to rescue this hematopoietic defect in Cbfb-deficient mice by introducing transgenes but found that whereas the introduction of Tie2-Cbfb induced erythro-myeloid progenitors (EMPs), only the introduction of Ly6a-Cbfb induced HSCs (Chen et al., 2011). These findings strongly suggest that hemogenic endothelial cells producing EMPs and HSCs are distinct.
In the yolk sac, EOMES transcription factor is expressed earlier than RUNX1, and regulates hematopoietic cell production through RUNX1 (Harland et al., 2021). Additionally, Meis1 is reported to be expressed before RUNX1 in the AGM region, and is important in regulating pre-hemogenic endothelium development (Coulombe et al., 2023). Meis1-knockout mice show reduced hematopoietic cells, including HSC production, and are embryonic lethal by E14.5 due to defective hematopoiesis and angiogenesis (Hisa et al., 2004; Azcoitia et al., 2005). Thus, studies suggest that fate determination from endothelial cells to hemogenic endothelial cells is initiated prior to the expression of RUNX1, and further elucidation of the mechanism underlying hemogenic endothelium development is necessary to expound upon these findings.
The search for hemogenic endothelium markers working alongside RUNX1 is ongoing (Fadlullah et al., 2022). Several groups are focusing on cell-surface markers to isolate select cells for experimentation. CD44 is expressed in endothelial cells, including hemogenic endothelial cells, and is also maintained in IAHCs formed by EHT from hemogenic endothelium (Oatley et al., 2020). Recently, it was reported that CD32 is characteristically expressed in hemogenic endothelial cells in human embryonic and human iPS-derived endothelial cells (Scarfo et al., 2024). Many CD32+ endothelial cells differentiate into hematopoietic cells, which enriches the hemogenic endothelium. However, it remains unclear whether CD32+ hemogenic endothelial cells can differentiate into HSCs, because an in vitro system that demonstrates the differentiation of human hemogenic endothelial cells into HSCs has not been established. Other works indicate the existence of different types of hemogenic endothelial cells (Chen et al., 2011; Dignum et al., 2021; Kobayashi et al., 2023), and thus we expect to see future work describing HSC-specific hemogenic endothelial cells.
Molecular mechanism of EHTEHT is based on two events: the loss of endothelial cell characteristics and the acquisition of hematopoietic cell characteristics. As IAHCs were not formed from hemogenic endothelium in Runx1-deficient mouse embryos (North et al., 1999; Yokomizo et al., 2001), many studies have since sought to investigate the role of RUNX1 in EHT. In an in vitro differentiation system using ES cells, one group showed that the loss of Gfi1 and Gfi1b—the target genes of RUNX1—results in the persistence of endothelial cell morphology instead of the spherical shape characteristic of hematopoietic cells (Lancrin et al., 2012). The same group has also shown that the loss of Gfi1 and Gfi1b in the AGM region prevents EHT and the formation of IAHCs, and thus a failure to produce HSCs (Thambyrajah et al., 2016). In their report, the authors showed that Gfi1 is specifically expressed in hemogenic endothelium from E10.5 and that its expression gradually decreases. However, contrastingly, the expression of Gfi1b increases, and this is consistent with the expression pattern of hematopoietic cell markers, such as KIT and CD41. The authors also show that a complex comprising LSD1 generates epigenetic changes that contribute to the loss of endothelial cell characteristics by GFI1 and GFI1B. Yet, despite these findings, inducing Gfi1 and Gfi1b expression in Runx1 KO cells to produce hematopoietic-like spherical cells leads to low colony-forming capacity. Collectively, these results suggest that molecules other than GFI1 and GFI1B might be involved in the acquisition of hematopoietic cell characteristics (Lancrin et al., 2012).
In parallel with the loss of endothelial cell characteristics by GFI1 and GFI1B, several hematopoietic-related genes become activated. ETS transcription factor Spi1 is one of the target genes activated by RUNX1 (Okada et al., 1998; Huang et al., 2008; Hoogenkamp et al., 2009). In Spi1-deficient mice, there is a reduction in the proportion of pre-HSCs (CD31+KIT+CD45+ cells) in the AGM region as well as HSCs in the fetal liver (Kim et al., 2004; Zhang et al., 2024). The requirement of SPI1 for the acquisition of hematopoietic competence is conserved across species. Single-cell RNA-sequencing (scRNA-seq) data analyses of the human fetal AGM region or of endothelial cells and hematopoietic cells derived from the human iPS cells show that the decrease in endothelial cell marker genes, Cdh5 and Sox17, is accompanied by an increase in Runx1, Spi1, and Gata2 (Qu et al., 2024). Spi1 regulates the heterogeneity of hematopoietic cell differentiation pathways, and the Spi1 target genes, Lyl1 and Klf1, determine the direction of hematopoietic cell differentiation (Qu et al., 2024).
GATA2 is another transcription factor that regulates EHT. Deletion of the transcriptional regulatory region of Gata2 can inhibit EHT and the formation of IAHCs (Gao et al., 2013). Interestingly, the authors show that repression of Gata2 transcription activity can suppress the expression of other transcription factors, such as Runx1 and Tal1. Previous reports showed that deletion of Tal1 causes embryonic lethality due to hematopoietic failure and TAL1 contributes to erythroid differentiation (Shivdasani et al., 1995; Mikkola et al., 2003). Thus, it is known that there are multiple transcription factors involved in EHT, and they cause EHT in a coordinated manner. This intricate network of transcription factors has been shown to continuously play a crucial role throughout the developmental process of HSCs (Wilson et al., 2010). On the other hand, studies using zebrafish and mice have also reported that GATA2 causes HSC development independently of RUNX1 (Bresciani et al., 2021). Gata2-knockout mice have been reported to die of hematopoietic failure at around E10.5 (Tsai et al., 1994), and Gata2 heterozygous knockout mice also show abnormalities in HSC generation and function (Ling et al., 2004). More interestingly, Gata2 loss specifically in Cdh5-expressing endothelial cells inhibits HSC generation, and Gata2 loss in Vav-expressing hematopoietic cells after EHT results in an inability to maintain HSCs. These reports suggest that GATA2 plays a dual role in the regulation of HSCs from their generation to their maintenance (de Pater et al., 2013). On the other hand, the deletion of Runx1 specifically in Vav-expressing hematopoietic cells does not reduce HSCs (Chen et al., 2009), suggesting that RUNX1 is important for differentiation progression up to EHT before E11.5.
Regarding EHT process, many scRNA-seq analyses have been conducted using pre- and post-EHT cells to explore its mechanisms and specific markers (Zhou et al., 2016; Baron et al., 2018; Hou et al., 2020; Vink et al., 2020; Zhu et al., 2020; Calvanese et al., 2022; Fadlullah et al., 2022; Hadland et al., 2022; Yokomizo et al., 2022; Menegatti et al., 2023). These analyses have confirmed the dynamic expression patterns of transcription factors such as Runx1 and Gfi1, which have previously been implicated in EHT. Furthermore, similarities between mice and humans have been observed in the expression of HSC-related transcription factors (Hlf and Mecom) (Zhou et al., 2016; Calvanese et al., 2022; Yokomizo et al., 2022). However, no mention has been made regarding the potential involvement of novel transcription factors in EHT. On the other hand, these studies have been highly effective in identifying new surface markers. Hadland et al. identified VE-cadherin+CD61+EPCR+ cells as a precursor to functional HSCs (Hadland et al., 2022), Vink et al. reported that the earliest functional HSCs are marked by CD27 (Vink et al., 2020), and Menegatti et al. identified CD82 as a novel surface marker specific to EHT (Menegatti et al., 2023).
Formation of IAHCs and their heterogeneityEHT causes the formation of specific cell clusters in arteries called IAHCs (Boisset et al., 2010; Yokomizo and Dzierzak, 2010). The cells constituting these IAHCs express the transcription factor Hlf, the expression of which is maintained after the cells differentiate into HSCs (Yokomizo et al., 2019). Hlf expression is regulated by the transcription factor Evi1, as evidenced by the reduction in Hlf-positive cells in Evi1-deficient mice. Thus, Evi1 activation and the subsequent expression of Hlf in cells within IAHCs may be a pathway for HSC differentiation. Interestingly, Evi1 expression is heterogeneous within IAHCs and cells with high Evi1 expression can differentiate into HSCs (Yokomizo et al., 2022). Therefore, it is possible that cells with high Evi1 expression within IAHCs are pre-HSCs. Future studies are expected to reveal the differentiation pathway of pre-HSCs to HSCs using a combination of transcription factors and cell-surface antigens as markers.
HSC development has been defined using cell-surface antigens (Figure 1). Hemogenic endothelial cells transform into hematopoietic cells by EHT, and demonstrate a significant change in cell-surface antigen expression. Hemogenic endothelium before EHT expresses the endothelial cell marker VE-cadherin and the arterial endothelial cell marker DLL4, while the blood cell markers KIT, CD41, and CD45 are not yet expressed (Hadland et al., 2015; Hadland et al., 2017; Morino-Koga et al., 2024). When EHT occurs and IAHCs are formed, DLL4 expression is downregulated (Porcheri et al., 2020) and this downregulation is inversely correlated with the increased expression of KIT and CD41. On the contrary, VE-cadherin expression is maintained after EHT (Rybtsov et al., 2011; Rybtsov et al., 2014; Porcheri et al., 2020; Morino-Koga et al., 2024). Cells that eventually become CD45+ and migrate to the liver will acquire the ability to become HSCs (Rybtsov et al., 2011; Rybtsov et al., 2014; Morino-Koga et al., 2024). After liver migration, VE-cadherin expression gradually decreases and HSCs expressing so-called HSC markers are detected (Morrison et al., 1995; Kim et al., 2005; Taoudi et al., 2005; Papathanasiou et al., 2009).
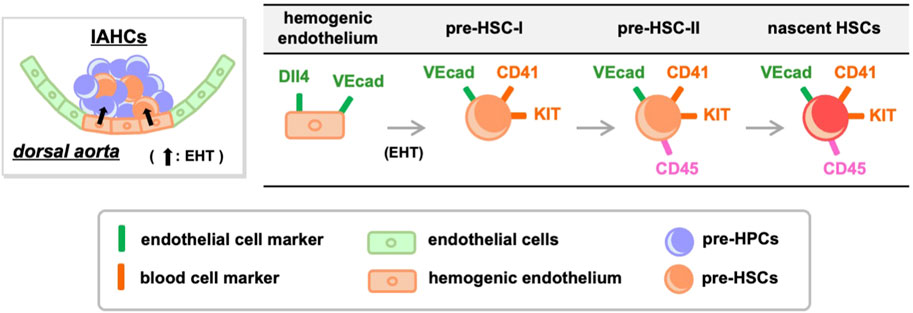
Figure 1. Transition of cell-surface antigens during HSC development. Hemogenic endothelium expressing endothelial cell markers undergoes EHT and differentiates into pre-HSC-I and pre-HPCs. During this process, some endothelial cell markers are retained while the expression of blood cell markers increases. Pre-HSC-I then differentiates into pre-HSC-II, which express CD45, and ultimately mature into HSCs. VEcad, VE-cadherin.
Of note, IAHCs are not a cell mass containing only pre-HSCs but also contain heterogeneous cell populations, most of which are pre-HPCs that do not differentiate into HSCs (Baron et al., 2018; Yokomizo et al., 2022). Consequently, it is difficult to distinguish pre-HSCs from pre-HPCs using the current definition of pre-HSCs through cell-surface antigens. The search for specific marker molecules for pre-HSCs is an upcoming challenge.
HSC development and signaling moleculesThus far, HSC development via the intricate interactions of nuclear transcription factors has been summarized. The next question is, how do extracellular signals control HSC development? Unlike transcription factors, it is challenging to clarify the roles of signals because knockout mice do not exhibit clear phenotypes. A common approach is to add signaling molecules to culture systems to observe their effects. Specifically, tissues or cell populations that are precursors to HSCs are isolated from mouse embryos and transferred to culture systems where different signaling molecules are introduced to assess whether transplantable HSCs can be induced. This method helps to infer the signaling pathways functioning in vivo.
SCFAfter studies showed evidence for HSCs within the AGM region, many groups sought to differentiate AGM-derived cells into HSCs in vitro. Medvinsky’s group developed an ex-vivo culture system to demonstrate the existence of pre-HSCs. They co-cultured pre-HSC candidate cells (VE-cadherin+CD45+ cells) with AGM stromal elements (including endothelial cells) in the presence of stem cell factor (SCF), Fms-related tyrosine kinase 3 ligand (Flt3l), and interleukin-3 (IL-3), and showed induction of transplantable HSCs (Taoudi et al., 2008). These AGM stromal elements could be replaced by simply using the OP9 stromal cell line (Rybtsov et al., 2011), with HSCs induced from E9.5 pre-HSC-I (VE-cadherin+CD45−CD41lo cells) (Rybtsov et al., 2014). This report also examined the necessity for signaling molecules, and showed that E9.5 pre-HSC-I differentiated into HSCs after supplementing SCF with OP9 stromal cells and serum, and this occurred even in the absence of other signaling molecules. Others have shown production of HSCs from E9.5 hemogenic endothelium under serum-free culture conditions following the addition of four signaling factors—SCF, Flt3l, IL-3, and thrombopoietin (TPO)—as well as AGM-derived endothelial feeder cells overexpressing Akt (Hadland et al., 2015). More recently, we showed that E11.5 pre-HSC-I differentiated into HSCs in the presence of SCF and TPO, even under serum-free and feeder-free conditions (Morino-Koga et al., 2024). These results indicate that SCF consistently contributes to HSC development from E9.5. KIT, an SCF receptor, is persistently expressed from E9.5 pre-HSC-I to E12.5 HSCs (Hadland et al., 2015; Morino-Koga et al., 2024), suggesting that SCF is continuously required for HSC development.
Scf deficiency is known to cause perinatal lethality due to severe anemia (Ding et al., 2012), and loss of Scf activity can impair HSC generation and function (Azzoni et al., 2018). Therefore, SCF might be important for HSC development and hematopoietic function. However, does SCF reside in the AGM region? Through scRNA-seq of periaortic tissues and livers from E10.5 and E11.5 mouse embryos, we recently revealed that Scf is expressed in various tissues, including endothelial cells, stromal cells, genital ridge progenitor cells, nephric duct, and hepatoblasts (Morino-Koga et al., 2024). These results are consistent with the earlier histological findings showing Scf expression to be strongly observed in the ventral wall of the dorsal aorta (Souilhol et al., 2016a). In that report, Scf expression was also detected in surrounding cells, such as stromal cells and genital ridge cells, supporting that Scf is present in the microenvironment where IAHC formation occurs. Among these, Scf produced by endothelial cells is important for HSC development (Azzoni et al., 2018).
Flt3lFlt3l was identified as a ligand for FLT3 (Lyman et al., 1993) and, since Flt3-deficient mice exhibit hematopoietic abnormalities (Mackarehtschian et al., 1995), it is thought to be involved in lineage commitment. Additionally, FLT3 is often used as a marker for hematopoietic progenitors immediately after differentiation from HSCs (Adolfsson et al., 2005; Forsberg et al., 2006). Tracing experiments using Flt3-Cre BAC transgenic mice have shown that nearly all hematopoietic lineages, except HSCs, are marked (Boyer et al., 2011; Buza-Vidas et al., 2011). Interestingly, a subset of HSCs in the embryonic stage is marked by Flt3-Cre, but these FLT3+ cell-derived HSCs disappear after birth (Beaudin et al., 2016). These results suggest that Flt3l/FLT3 signaling is not essential for the development and maintenance of the types of HSCs that are preserved into adulthood. On the other hand, in HSC induction culture systems, Flt3l is added along with SCF and IL-3 and is used for the induction of HSCs from pre-HSCs after E9.5 (Rybtsov et al., 2011; Rybtsov et al., 2014). In an aggregation culture system developed later using OP9 stromal cells, the authors suggested that Flt3l was not needed (Rybtsov et al., 2014) and suggested that Flt3l may not be essential for the in vitro development (maturation) of HSCs. Indeed, it is possible that the same effect afforded by Flt3l is compensated by other pathways.
IL-3Early findings by Robin et al. highlighted IL-3 as an inducer of transplantable HSCs in an in vitro culture system using E11.5 AGM region isolated from Runx1+/− mice (Robin et al., 2006). Since then, IL-3 is touted to play a role in HSC development through RUNX1. In the same report, the authors also showed that lL-3 was expressed in the cells of the AGM region, with expression of the IL-3 receptor noted on some hematopoietic cells in that region. Later work showed IL-3 as an effective inducer of HSC differentiation of E11.5 pre-HSCs in a co-aggregation culture with OP9 stromal cells (Rybtsov et al., 2014). Collectively, these findings suggest a contribution by IL-3 in the differentiation of pre-HSCs into HSCs after E11.5.
Inflammatory signalingInflammatory signaling has been reported to be involved in the development and maintenance of HSCs during embryogenesis (Espin-Palazon et al., 2014; Sawamiphak et al., 2014; He et al., 2015; Mariani et al., 2019; Frame et al., 2020; Zhang et al., 2024). Both Interferon-γ (Ifn-γ)- and Ifn-γ receptor-deficient mouse embryos show a reduced proportion of functional HSCs (Li et al., 2014). However, bone marrow-derived HSCs in Ifn-γ-deficient mice are normal (Baldridge et al., 2010), suggesting that the HSCs produced are functional. It is surmised that the addition of IFN-γ may be effective in inducing functional HSCs in vitro. One group showed that the addition of interferon-α (IFN-α) to AGM-derived cells using an in vitro culture system could induce HSCs with high engraftment capacity in the bone marrow (Kim et al., 2016). In that report, Ifn-α was minimally expressed in the E11.5 AGM region but upregulated in the E13.5 fetal liver, suggesting IFN-α involvement in the maturation of HSCs in the fetal liver.
Notch signalingRecently, we reported that E11.5 pre-HSC-I could induce HSCs by adding only SCF and TPO even in the absence of serum and feeder cells (Morino-Koga et al., 2024). However, we also showed that E10.5 pre-HSC-I and E10.5 hemogenic endothelium could not differentiate into HSCs with SCF and TPO, requiring co-culture with the endothelial cell line. These results are consistent with previous results obtained using Akt-overexpressing embryonic endothelial cell lines (Hadland et al., 2015; Hadland et al., 2017), suggesting that co-culture with endothelial cells might be necessary to induce HSCs from E10.5 hemogenic endothelium in an in vitro culture system. Furthermore, HSCs have been successfully produced from E9.5-E10.5 CD45−VE-cadherin+CD41-Dll4+ cells, which are defined as arterial endothelial cells, by co-culturing with endothelial feeder cells (Hadland et al., 2017; Morino-Koga et al., 2024). Thus, it might support the process of generating HSCs from arterial endothelial cells via EHT.
What kind of environment do endothelial cells provide for hemogenic endothelium? One of the most promising candidates is Notch signaling (Clements et al., 2011; Kanz et al., 2016; Rho et al., 2019; Thambyrajah and Bigas, 2022). Notch signaling is also important for angiogenesis, and thus it is not easy to distinguish whether hematopoietic cells are directly affected or if their absence is due to a defect in angiogenesis. Studies using ES cells or endothelial cells derived from mice lacking Notch1 have reported that Notch1 is required for endothelial cells to undergo EHT and differentiate into HSCs (Kumano et al., 2003; Hadland et al., 2004). These results suggest that hemogenic endothelium and/or pre-HSCs might express Notch1, since cells lacking Notch1 cannot differentiate into HSCs. HSCs can be induced from E10.5 CD45−VE-cadherin+ cells with activated Notch signaling by aggregate culture with OP9 stromal cells and suppressed by either adding the Notch inhibitor DAPT to the AGM tissue culture at E10.5, or by treating a reaggregated culture of E10.5 AGM-derived cells with a Notch1 inhibitor antibody (Souilhol et al., 2016b). Collectively, these findings suggest that Notch1-expressing cells differentiate into HSCs. Let us return to the main question: Does the endothelial cell line express a ligand acting on the Notch1 receptor? Whole-embryo immunohistochemical staining of mice at E9.5 and E10.5 shows that endothelial cells in the P-Sp/AGM region express the Notch ligands (DLL4, JAG1, and JAG2) and receptors (Notch1 and Notch4) (Robert-Moreno et al., 2005). Notch1 is expressed in the ventral side of the dorsal aorta where IAHCs form, whereas Notch4 is uniformly expressed throughout the vessel. Therefore, these Notch1-expressing cells are expected to undergo EHT and differentiate into HSCs. On the other hand, all three Notch ligands are expressed throughout the endothelial cells. Compared with primary endothelial cells, the authors highlighted an upregulation in the expression of DLL1, DLL4, JAG1, and JAG2 in Akt-overexpressing endothelial cells (Hadland et al., 2015). In addition, endothelial cell lines that contributed to HSC development showed expression of Dll1, Dll4, and Jag1, but not Jag2; albeit the expression intensity varied (Morino-Koga et al., 2024). Thus, the endothelial cell line may not only serve as a scaffold but may also contribute to the HSC differentiation via the Notch ligands, DLL4 and JAG1.
For Notch signaling, we analyzed the scRNA-seq dataset using endothelial cells and hematopoietic cells from E10.5-E11.5 mouse embryos (Figure 2) (Yokomizo et al., 2022; Morino-Koga et al., 2024). Consistent with previous histological findings (Robert-Moreno et al., 2005), expression of Dll4, Jag1, and Jag2 was observed in arterial endothelial cells, but not Dll1 expression. Furthermore, Notch4, which is reported to be uniformly expressed in vascular endothelial cells, was highly expressed in endothelial cells. Interestingly, Notch1 was expressed not only in endothelial cells but also in regions reported to be hemogenic endothelium and in pre-HSCs, consistent with previous reports that Notch1 is expressed in HSC precursors (Kumano et al., 2003; Hadland et al., 2004; Souilhol et al., 2016b). Thus, in the developmental environment of HSCs—particularly in the AGM region of E10.5-E11.5 where pre-HSC-I is produced—the innermost layer, the arterial endothelium, expresses the Notch ligand. Although Notch signaling remains important, Hadland et al. recently reported on the contributions of factors other than Notch signaling in the role of the stromal environment within the in vitro HSC induction system (Hadland et al., 2022). This report demonstrated that Notch signaling alone could not induce HSCs and that additional signal activation from fibronectin and CXCL12 was required, supporting that multiple signaling molecules are coordinately involved in HSC development.
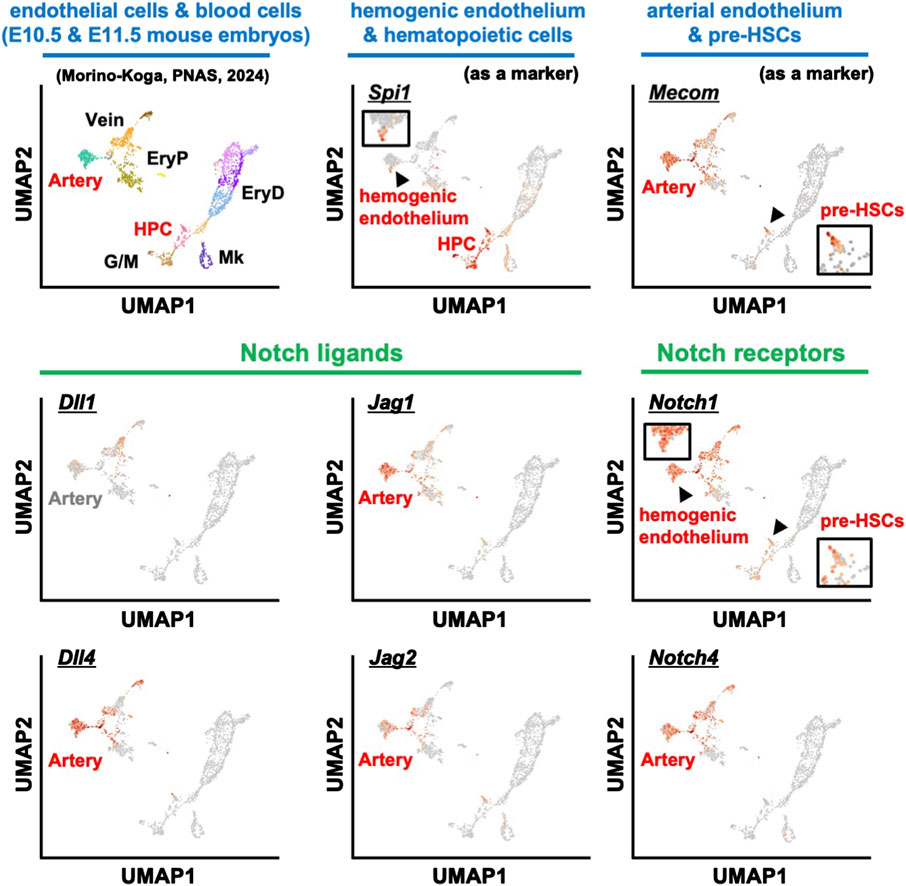
Figure 2. scRNA-seq analysis of blood and endothelial cells from E10.5 and E11.5 mouse embryos. The expression patterns of various marker genes: hemogenic endothelium and HPC (Spi1), endothelial cells and pre-HSCs (Mecom), Notch ligands (Dll1, Dll4, Jag1, Jag2), and Notch receptors (Notch1 and Notch4). HPC, hematopoietic progenitor cells; EryP, primitive erythrocytes; EryD, definitive erythrocytes; G/M, granulocytes and monocytes; Mk, megakaryocytes.
BMP4BMP4 was originally identified as being important for mesoderm development (Winnier et al., 1995; Lengerke et al., 2008) and in the promotion of EHT. BMP4 can generate hematopoietic cells from endothelial cells using an in vitro differentiation assay (Tsuruda et al., 2021). Furthermore, cells with activated BMP signaling in the AGM region are present in IAHCs and there is evidence that transplantation of BMP-activated cells into irradiated mice results in the engraftment of HSCs (Crisan et al., 2015).
BMP4 is abundant on the ventral side of the dorsal aorta from E10.5-E11.5 (Durand et al., 2007), while the expression of BMP inhibitory molecules is increased, and indeed, BMP signaling inhibitors have been shown to promote HSC development in an in vitro culture system (Souilhol et al., 2016a; McGarvey et al., 2017). Recently, we found that HSCs were induced by adding BMP4, SCF, and TPO to E9.5 hemogenic endothelium using an in vitro culture system (Tsuruda et al., 2024). In this culture system, it was important to remove BMP4 in the latter part of the culture. On the other hand, BMP4 was not necessary when generating HSCs from E10.5 hemogenic endothelium or pre-HSC-I (Morino-Koga et al., 2024). Therefore, it is likely that BMP4 is required for HSC development before E10.5 and gradually becomes unnecessary.
Retinoic acid (RA)In mouse embryos deficient in the RA synthase enzyme Raldh2, RA synthesis is impaired, resulting in defective hemogenic endothelium development in the yolk sac and decreased hematopoietic cellularity (Goldie et al., 2008). Raldh2-deficient mouse embryos are embryonic lethal at around E10.5 due to various morphogenetic abnormalities (Niederreither et al., 1999). The deletion of Raldh2 specifically in Cdh5-expressing endothelial cells inhibits HSC production (Chanda et al., 2013). In that report, Cdh5-expressing cells at E10.5-E11.5 were shown to express the RA receptor, and treatment of Raldh2-deficient mice with an RA receptor agonist restored HSC production. Therefore, it is expected that supplementation with RA can promote HSC production in vitro.
Sonic hedgehog (Shh)The notochord is present in the dorsal periphery of the dorsal aorta. Shh supplied by the notochord is important for the differentiation of E10.5 AGM-derived cells into HSCs but not E11.5 AGM-derived cells (Souilhol et al., 2016a). Indeed, since the addition of Shh to the tissue culture systems using the E10 AGM region differentiates them into HSCs (Peeters et al., 2009), it is still unclear whether Shh is required before E9.5. Importantly, strong Shh signaling was observed in stromal cells located around the dorsal aorta (Peeters et al., 2009; Souilhol et al., 2016a). It is possible that Shh may not act directly on HSC progenitor cells but may indirectly influence HSC development by acting on surrounding cells.
CatecholamineThe nervous system surrounding the AGM region is known to influence HSC development (Kwan et al., 2016; Lv et al., 2017). Cells of the sympathetic nervous system developing near the aorta secrete catecholamines via the transcription factor Gata3, which promotes HSC development in the AGM region (Fitch et al., 2012). The cell cycle regulator p57Kip2 (Cdkn1c) is also a regulator of the sympathetic nervous system; its suppression promotes sympathetic nervous system development that, in turn, promotes HSC development via catecholamines (Mascarenhas et al., 2009; Kapeni et al., 2022). Although experiments adding catecholamines have been studied in tissue culture of the E11.5 AGM region and contribute to HSC production (Fitch et al., 2012), it is unclear when catecholamines begin to affect HSC development. Further insight could be gained by additional experiments in an in vitro differentiation system of hemogenic endothelium or pre-HSCs.
TPOTPO signaling has been reported to be important for HSC maintenance and quiescence in the bone marrow and is also required for HSC engraftment capacity (Qian et al., 2007; Yoshihara et al., 2007). Yet, the source of Tpo is the liver, not the bone marrow (Decker et al., 2018); this suggests that TPO expression in the liver is important in maintaining HSCs. However, since HSC development in the fetal liver in Tpo-deficient mice is normal (Qian et al., 2007; Lee et al., 2022), evidence suggests that TPO is not required for HSC development in the fetal liver.
On the other hand, many reports have shown that TPO is important in differentiating hemogenic endothelium and pre-HSCs into HSCs in vitro culture (Kieusseian et al., 2012; Hadland et al., 2015; Mascarenhas et al., 2016). Recently, we reported that E11.5 pre-HSC-I can be induced into HSCs in an in vitro culture system without serum or feeder cells using just two signaling molecules: SCF and TPO (Morino-Koga et al., 2024). Furthermore, the cell-surface expression of MPL, the receptor for TPO, was gradually observed on E10.5 pre-HSC-I. Considering that HSC engraftment capacity was not very high, these two signaling molecules are necessary but insufficient for HSC differentiation. Interestingly, no Tpo-expressing cells were present in the AGM environment or surrounding tissues, and hepatoblasts in the fetal liver were the only Tpo-producing cells in the HSC developmental environment (Morino-Koga et al., 2024). HSCs are first produced at the AGM region but are detectable in high quantities at E12.5 in the fetal liver, suggesting that pre-HSCs can mature by migrating to the fetal liver and activating TPO signaling. As mentioned above, HSCs were produced even when TPO signaling was lost, suggesting the existence of compensatory mechanisms that activate the TPO downstream molecule, JAK2.
ConclusionThis review focused on signaling molecules in vitro differentiation systems in HSC development (Figure 3). Meanwhile, efforts are currently underway to identify transcription factors and signaling molecules that control HSC development and their engraftment in the bone marrow using human iPS cells (Doulatov et al., 2013; Piau et al., 2023; Ng et al., 2024). We believe that establishing an HSC in vitro differentiation system is important because it will lead to clinical applications of HSCs for hematopoietic disorders. However, in addition to the signaling molecules shown in this review, multiple environmental factors surrounding the AGM region are likely to support HSC development. For example, studies using mice and zebrafish show that HSC development is affected by blood flow-induced shear stress (Adamo et al., 2009; North et al., 2009). Therefore, it is crucial to investigate in detail which signaling molecules affect HSC development when recreating the microenvironment in vitro. Our serum-free culture system using only commercially available products (Morino-Koga et al., 2024) allows for the re-evaluation of key signaling molecules during HSC development under serum-containing conditions or using tissue culture systems derived from the AGM region. In the future, the identification of truly essential signaling molecules is expected to contribute to the establishment of in vitro differentiation systems for HSCs.
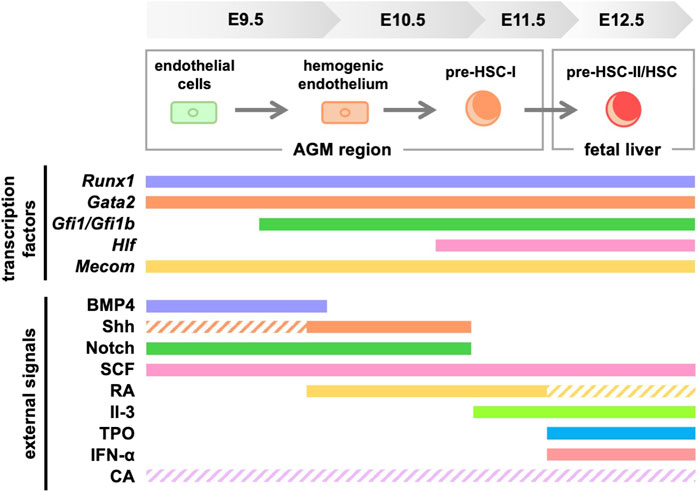
Figure 3. Signaling molecules and transcription factors required for HSC generation in mice. Solid lines indicate the signaling molecules involved in development, while dashed lines indicate those with unclear involvement. RA, retinoic acid; TPO, thrombopoietin; CA, catecholamine.
Author contributionsSM-K: Writing–original draft. TY: Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS Kakenhi Grant (JP21K08398, JP24K11519, JP23K27632, JP23K18305), the Chemo-Sero-Therapeutic Research Institute (to TY), Astellas Foundation for Research on Metabolic Disorders (to TY), the program of the Joint Usage/Research Center for Developmental Medicine and High Depth Omics, Institute of Molecular Embryology and Genetics (IMEG), Kumamoto University (to SM-K and TY), and the program of the Inter-University Research Network for High Depth Omics, IMEG, Kumamoto University (to SM-K).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsIAHC, intra-aortic hematopoietic cluster; HSC, hematopoietic stem cell; EMP, erythro-myeloid progenitor; EHT, endothelial-to-hematopoietic transition; pre-HSC, hematopoietic stem cell precursor; AGM, aorta-gonad-mesonephros.
ReferencesAdamo, L., Naveiras, O., Wenzel, P. L., McKinney-Freeman, S., Mack, P. J., Gracia-Sancho, J., et al. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature 459 (7250), 1131–1135. doi:10.1038/nature08073
PubMed Abstract | CrossRef Full Text | Google Scholar
Adolfsson, J., Mansson, R., Buza-Vidas, N., Hultquist, A., Liuba, K., Jensen, C. T., et al. (2005). Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121 (2), 295–306. doi:10.1016/j.cell.2005.02.013
PubMed Abstract | CrossRef Full Text | Google Scholar
Azcoitia, V., Aracil, M., Martinez, A. C., and Torres, M. (2005). The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev. Biol. 280 (2), 307–320. doi:10.1016/j.ydbio.2005.01.004
PubMed Abstract | CrossRef Full Text | Google Scholar
Azzoni, E., Frontera, V., McGrath, K. E., Harman, J., Carrelha, J., Nerlov, C., et al. (2018). Kit ligand has a critical role in mouse yolk sac and aorta-gonad-mesonephros hematopoiesis. EMBO Rep. 19 (10), e45477. doi:10.15252/embr.201745477
PubMed Abstract | CrossRef Full Text | Google Scholar
Baldridge, M. T., King, K. Y., Boles, N. C., Weksberg, D. C., and Goodell, M. A. (2010). Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 465 (7299), 793–797. doi:10.1038/nature09135
PubMed Abstract | CrossRef Full Text | Google Scholar
Baron, C. S., Kester, L., Klaus, A., Boisset, J. C., Thambyrajah, R., Yvernogeau, L., et al. (2018). Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat. Commun. 9 (1), 2517. doi:10.1038/s41467-018-04893-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Beaudin, A. E., Boyer, S. W., Perez-Cunningham, J., Hernandez, G. E., Derderian, S. C., Jujjavarapu, C., et al. (2016). A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell 19 (6), 768–783. doi:10.1016/j.stem.2016.08.013
PubMed Abstract | CrossRef Full Text | Google Scholar
Boisset, J. C., Clapes, T., Klaus, A., Papazian, N., Onderwater, J., Mommaas-Kienhuis, M., et al. (2015). Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood 125 (3), 465–469. doi:10.1182/blood-2014-07-588954
PubMed Abstract | CrossRef Full Text | Google Scholar
Boisset, J. C., van Cappellen, W., Andrieu-Soler, C., Galjart, N., Dzierzak, E., and Robin, C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464 (7285), 116–120. doi:10.1038/nature08764
PubMed Abstract | CrossRef Full Text | Google Scholar
Boyer, S. W., Schroeder, A. V., Smith-Berdan, S., and Forsberg, E. C. (2011). All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 9 (1), 64–73. doi:10.1016/j.stem.2011.04.021
PubMed Abstract | CrossRef Full Text | Google Scholar
Bresciani, E., Carrington, B., Yu, K., Kim, E. M., Zhen, T., Guzman, V. S., et al. (2021). Redundant mechanisms driven independently by RUNX1 and GATA2 for hematopoietic development. Blood Adv. 5 (23), 4949–4962. doi:10.1182/bloodadvances.2020003969
PubMed Abstract | CrossRef Full Text | Google Scholar
Buza-Vidas, N., Woll, P., Hultquist, A., Duarte, S., Lutteropp, M., Bouriez-Jones, T., et al. (2011). FLT3 expression initiates in fully multipotent mouse hematopoietic progenitor cells. Blood 118 (6), 1544–1548. doi:10.1182/blood-2010-10-316232
PubMed Abstract | CrossRef Full Text | Google Scholar
Calvanese, V., Capellera-Garcia, S., Ma, F., Fares, I., Liebscher, S., Ng, E. S., et al. (2022). Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature 604 (7906), 534–540. doi:10.1038/s41586-022-04571-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Chanda, B., Ditadi, A., Iscove, N. N., and Keller, G. (2013). Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 155 (1), 215–227. doi:10.1016/j.cell.2013.08.055
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, M. J., Li, Y., De Obaldia, M. E., Yang, Q., Yzaguirre, A. D., Yamada-Inagawa, T., et al. (2011). Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9 (6), 541–552. doi:10.1016/j.stem.2011.10.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E., and Speck, N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457 (7231), 887–891. doi:10.1038/nature07619
PubMed Abstract | CrossRef Full Text | Google Scholar
Clements, W. K., Kim, A. D., Ong, K. G., Moore, J. C., Lawson, N. D., and Traver, D. (2011). A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474 (7350), 220–224. doi:10.1038/nature10107
PubMed Abstract | CrossRef Full Text | Google Scholar
Coulombe, P., Cole, G., Fentiman, A., Parker, J. D. K., Yung, E., Bilenky, M., et al. (2023). Meis1 establishes the pre-hemogenic endothelial state prior to Runx1 expression. Nat. Commun. 14 (1), 4537. doi:10.1038/s41467-023-40283-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Crisan, M., Kartalaei, P. S., Vink, C. S., Yamada-Inagawa, T., Bollerot, K., van, I. W., et al. (2015). BMP signalling differentially regulates distinct haematopoietic stem cell types. Nat. Commun. 6, 8040. doi:10.1038/ncomms9040
PubMed Abstract | CrossRef Full Text | Google Scholar
Cumano, A., Dieterlen-Lievre, F., and Godin, I. (1996). Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86 (6), 907–916. doi:10.1016/s0092-8674(00)80166-x
PubMed Abstract | CrossRef Full Text | Google Scholar
de Bruijn, M. F., Ma, X., Robin, C., Ottersbach, K., Sanchez, M. J., and Dzierzak, E. (2002). Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16 (5), 673–683. doi:10.1016/s1074-7613(02)00313-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Decker, M., Leslie, J., Liu, Q., and Ding, L. (2018). Hepatic thrombopoietin is required for bone marrow hematopoietic stem cell maintenance. Science 360 (6384), 106–110. doi:10.1126/science.aap8861
PubMed Abstract | CrossRef Full Text | Google Scholar
de Pater, E., Kaimakis, P., Vink, C. S., Yokomizo, T., Yamada-Inagawa, T., van der Linden, R., et al. (2013). Gata2 is required for HSC generation and survival. J. Exp. Med. 210 (13), 2843–2850. doi:10.1084/jem.20130751
PubMed Abstract | CrossRef Full Text | Google Scholar
Dignum, T., Varnum-Finney, B., Srivatsan, S. R., Dozono, S., Waltner, O., Heck, A. M., et al. (2021). Multipotent progenitors and hematopoietic stem cells arise independently from hemogenic endothelium in the mouse embryo. Cell Rep. 36 (11), 109675. doi:10.1016/j.celrep.2021.109675
PubMed Abstract | CrossRef Full Text | Google Scholar
Ding, L., Saunders, T. L., Enikolopov, G., and Morrison, S. J. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481 (7382), 457–462. doi:10.1038/nature10783
Comments (0)